10-Q: Quarterly report pursuant to Section 13 or 15(d)
Published on August 11, 2022
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________
FORM
______________________________________
(Mark One)
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
or
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number:
______________________________________
(Exact name of registrant as specified in its charter)
______________________________________
Québec, |
|
(State or other jurisdiction of |
(I.R.S. Employer |
(Address of principal executive offices, including zip code)
(Registrant’s telephone number, including area code)
______________________________________
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
|
☒ |
|
Smaller reporting company |
|
||
Emerging growth company |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes
The number of outstanding common shares of the registrant, no par value per share, as of August 11, 2022, was
ACASTI PHARMA INC.
QUARTERLY REPORT ON FORM 10-Q
For the Quarter Ended June 30, 2022
Table of Contents
|
|
Page |
|
||
7 |
||
|
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
19 |
|
|
|
|
Item 3. |
Quantitative and Qualitative Disclosures About Market Risk |
36 |
|
|
|
Item 4. |
Controls and Procedures |
36 |
|
|
|
|
||
37 |
||
|
|
|
37 |
||
|
|
|
37 |
||
|
|
|
37 |
||
|
|
|
37 |
||
|
|
|
37 |
||
|
|
|
38 |
||
2
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This quarterly report contains information that may be forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and forward-looking information within the meaning of Canadian securities laws, both of which we refer to in this quarterly report as forward-looking statements. Forward-looking statements can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking statements in this quarterly report include, among other things, information or statements about:
Although the forward-looking statements in this quarterly report are based upon what we believe are reasonable assumptions, you should not place undue reliance on those forward-looking statements since actual results may vary materially from them. Important assumptions made by us when making forward-looking statements include, among other things, assumptions by us that:
3
In addition, the forward-looking statements in this quarterly report are subject to a number of known and unknown risks, uncertainties and other factors many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking statements, including, among others:
4
5
All of the forward-looking statements in this quarterly report are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition, or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this quarterly report.
We express all amounts in this quarterly report in U.S. dollars, except where otherwise indicated. References to “$” and “U.S.$” are to U.S. dollars and references to “C$” or “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this quarterly report to “Acasti,” “the Corporation,” “we,” “us” and “our” refer to Acasti Pharma Inc. and its consolidated subsidiaries, including Acasti Pharma U.S., which is formerly Grace.
6
PART I. FINANCIAL INFORMATION
Item 1: Financial Information
Unaudited Condensed Consolidated Interim Financial Statements
7
Condensed Consolidated Interim Financial Statements of
(Unaudited)
ACASTI PHARMA INC.
Three Months ended June 30, 2022 and 2021
8
ACASTI PHARMA INC.
Condensed Consolidated Interim Balance Sheet
(Unaudited)
|
|
|
|
June 30, |
|
|
March 31, |
|
||
(Expressed in thousands of U.S. dollars except share data) |
|
Notes |
|
$ |
|
|
$ |
|
||
Assets |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
|
|
|
|
|
||
Short-term investments |
|
5 |
|
|
|
|
|
|
||
Receivables |
|
|
|
|
|
|
|
|
||
Assets held for sale |
|
6 |
|
|
|
|
|
|
||
Prepaid expenses |
|
|
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Right of use asset |
|
|
|
|
|
|
|
|
||
Equipment |
|
|
|
|
|
|
|
|
||
Intangible assets |
|
4 |
|
|
|
|
|
|
||
Goodwill |
|
4 |
|
|
|
|
|
|
||
Total assets |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Liabilities and shareholders’ equity |
|
|
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
|
|
||
Trade and other payables |
|
|
|
|
|
|
|
|
||
Lease liability |
|
|
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Derivative warrant liabilities |
|
|
|
|
|
|
|
|
||
Lease Liability |
|
|
|
|
|
|
|
|
||
Deferred tax liability |
|
|
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Shareholders’ equity: |
|
|
|
|
|
|
|
|
||
Common shares |
|
4,7(a) |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
|
|
||
Accumulated other comprehensive loss |
|
|
|
|
( |
) |
|
|
( |
) |
Accumulated deficit |
|
|
|
|
( |
) |
|
|
( |
) |
Total shareholder’s equity |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
|
12 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
||
Total liabilities and shareholders’ equity |
|
|
|
|
|
|
|
|
||
See accompanying notes to unaudited Interim financial statements.
9
ACASTI PHARMA INC.
Condensed Consolidated Interim Statements of Loss and Comprehensive Loss
(Unaudited)
Three Months ended June 30, 2022 and 2021
|
|
|
|
Three-month ended |
|
|||||
|
|
|
|
June 30, |
|
|
June 30, |
|
||
(Expressed in thousands of U.S dollars, except per share data) |
|
Notes |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
|
|
||
Operating expenses |
|
|
|
|
|
|
|
|
||
Research and development expenses, net of government assistance |
|
8 |
|
|
( |
) |
|
|
( |
) |
General and administrative expenses |
|
|
|
|
( |
) |
|
|
( |
) |
Sales and marketing expenses |
|
|
|
|
( |
) |
|
|
|
|
Loss from operating activities |
|
|
|
|
( |
) |
|
|
( |
) |
Financial income (expenses) |
|
9 |
|
|
( |
) |
|
|
|
|
Loss before income tax recovery |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
||
Income tax recovery |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Net loss and total comprehensive loss |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
||
Basic and diluted loss per share |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
||
Weighted average number of shares outstanding |
|
|
|
|
|
|
|
|
||
See accompanying notes to unaudited interim financial statements
10
ACASTI PARMA INC.
Condensed Consolidated Interim Statements of Changes in Shareholder’s Equity
(Unaudited)
Three Months ended June 30, 2022 and 2021
|
|
|
|
|
Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
(Expressed in thousands of U.S. dollars except share data) |
|
Notes |
|
|
Number |
|
|
Dollar |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
|||||||
|
|
|
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||
Balance, March 31, 2022 |
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
Net loss and total comprehensive loss for the period |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Cumulative translation adjustment |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Net proceeds from shares issued under the at-the-market (ATM) program |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Stock based compensation |
|
|
10 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Balance at June 30, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
(Expressed in thousands of US dollars except for share data) |
|
Notes |
|
Number |
|
|
Dollar |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
||||||
|
|
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
Balance, March 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
Net loss and total comprehensive loss for the period |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Cumulative translation adjustment |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Stock based compensation |
|
10 |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Balance at June 30, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
11
ACASTI PHARMA INC.
Condensed Consolidated Interim Statements of Cash Flows
(Unaudited)
Three Months ended June 30, 2022 and 2021
|
|
|
|
Three Months ended |
|
|||||
|
|
|
|
June 30, |
|
|
June 30, |
|
||
(thousands of U.S. dollars) |
|
Notes |
|
$ |
|
|
$ |
|
||
Cash flows used in operating activities: |
|
|
|
|
|
|
|
|
||
Net loss for the period |
|
|
|
|
( |
) |
|
|
( |
) |
Adjustments: |
|
|
|
|
|
|
|
|
||
Depreciation of equipment |
|
|
|
|
|
|
|
|
||
Stock-based compensation |
|
10 |
|
|
|
|
|
|
||
Change in fair value of warrant liabilities |
|
|
|
|
( |
) |
|
|
( |
) |
Income tax recovery |
|
|
|
|
( |
) |
|
|
|
|
Unrealized foreign exchange (gain) loss |
|
|
|
|
( |
) |
|
|
|
|
Changes in non-cash working capital items |
|
11 |
|
|
( |
) |
|
|
( |
) |
Net cash used in operating activities |
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
||
Cash flows from (used in) investing activities: |
|
|
|
|
|
|
|
|
||
Acquisition of equipment |
|
|
|
|
( |
) |
|
|
|
|
Acquisition of short-term investments |
|
|
|
|
( |
) |
|
|
( |
) |
Maturity of short-term investment |
|
|
|
|
|
|
|
|
||
Net cash from (used in) investing activities |
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
||
Cash flows from (used in) financing activities: |
|
|
|
|
|
|
|
|
||
Net proceeds from issuance of common shares under the at-the-market (ATM) |
|
(7a) |
|
|
|
|
|
|
||
Net cash from (used in) financing activities |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Effect of exchange rate fluctuations on cash and cash equivalents |
|
|
|
|
( |
) |
|
|
( |
) |
Translations effects on cash and cash equivalents related to reporting currency |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Net increase (decrease) in cash and cash equivalents |
|
|
|
|
|
|
|
( |
) |
|
Cash and cash equivalents, beginning of period |
|
|
|
|
|
|
|
|
||
Cash and cash equivalents, end of period |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Cash and cash equivalents are comprised of: |
|
|
|
|
|
|
|
|
||
Cash |
|
|
|
|
|
|
|
|
||
Cash equivalents |
|
|
|
|
|
|
|
|
||
See accompanying notes to unaudited interim financial statements.
12
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three Months ended June 30, 2022 and 2021
1. Nature of operation
Acasti Pharma Inc. (“Acasti” or the “Corporation”) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 3009 boul. de la Concorde East, Suite 102, Laval, Québec, Canada H7E 2B5.
In August 2021, the Corporation completed the acquisition via a share-for-share merger of Grace Therapeutics, Inc. (“Grace”), a privately held emerging biopharmaceutical company focused on developing innovative drug delivery technologies for the treatment of rare and orphan diseases. The post-merger Corporation is focused on building a late-stage specialty pharmaceutical company specializing in rare and orphan diseases and developing and commercializing products that improve clinical outcomes using our novel drug delivery technologies. The Corporation seeks to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by the Corporation for further development may be already approved in the target indication or could be repurposed for use in new indications.
The Corporation has incurred operating losses and negative cash flows from operations in each year since its inception. The Corporation expects to incur significant expenses and continued operating losses for the foreseeable future. The Corporation expects its expenses will increase substantially in connection with its ongoing activities, particularly as it advances clinical development for the first three drug candidates in the Corporation’s pipeline; continues to engage contract manufacturing organizations (“CMOs”) to manufacture its clinical study materials and to ultimately develop large-scale manufacturing capabilities in preparation for commercial launch; seeks regulatory approval for its drug candidates; and adds personnel to support its drug product development and future drug product launch and commercialization.
The Corporation does not expect to generate revenue from product sales unless and until it successfully completes drug development and obtains regulatory approval, which the Corporation expects will take several years and is subject to significant uncertainty. To date, the Corporation has financed its operations primarily through public offerings and private placements of its common shares, warrants and convertible debt and the proceeds from research tax credits. Until such time that the Corporation can generate significant revenue from drug product sales, if ever, it will require additional financing, which is expected to be sourced from a combination of public or private equity or debt financings or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties. Arrangements with collaborators or others may require the Corporation to relinquish certain rights related to its technologies or drug product candidates. Adequate additional financing may not be available to the Corporation on acceptable terms, or at all. The Corporation’s inability to raise capital as and when needed would have a negative impact on its financial condition and its ability to pursue its business strategy.
The Corporation remains subject to risks similar to other development stage companies in the biopharmaceutical industry, including compliance with government regulations, protection of proprietary technology, dependence on third party contractors and consultants and potential product liability, among others.
Reverse stock split
On August 26, 2021, the shareholders of the Corporation approved a resolution to undertake a reverse split of the common stock within a range of 1-6 to 1-8 with such specific ratio to be approved by the Acasti Board. All references in these financial statements to number of common shares, warrants and options, price per share and weighted average number of shares outstanding prior to the reverse split have been adjusted to reflect the approved reverse stock split of 1-
2. Summary of significant accounting policies:
Basis of presentation
These unaudited Consolidated Interim Financial Statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and on a basis consistent with those accounting principles followed by the Corporation and disclosed in note 2 of its most recent Annual Consolidated Financial Statements, except as disclosed in note 3 – Recent accounting pronouncements and policies and should be read in conjunction with such statements and notes thereto.
Functional currency
On April 1, 2022, the Corporation’s functional currency was changed from the Canadian dollar to the US dollar. This change is reflected prospectively in the Corporation’s financial statements.
FASB ASC Topic 830, “Functional Currency Matters,” requires a change in functional currency to be reported as of the date it is determined there has been a change, and it is generally accepted practice that the change is made at the start of the most recent period that approximates the date of the change. Management determined it would enact this change effective on April 1, 2022. While the change was based on a factual assessment, the determination of the date of the change required management’s judgement given the change in the Corporations primary economic and business environment, which has evolved over time. As part of management’s functional currency assessment, changes in economic facts and circumstances were considered. This included analysis of changes in: impact of the merger with Grace Therapeutics, management of operations, and in the composition of cash and short term investment
13
balances. Additionally, budgeting is in USD, whereas this was previously performed in CAD. The Corporations cash outflows consist primarily of USD cash balances and less of CAD, as also reflected in the budget.
Use of estimates
The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities (note 7), stock-based compensation (note 10), assets held for sale (note 6), supply agreement (note 12), valuation of intangibles (note 4). Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date, including whether contingencies should be accrued for, as well as in determining which research and development expenses qualify for investment tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and, therefore, could be different from the amounts recorded.
3. Recent accounting pronouncements
The Corporation has considered recent accounting pronouncements and concluded that they are either not applicable to the business or that the effect is not expected to be material to the consolidated financial statements as a result of future adoption.
4. Intangible assets
On August 27, 2021, the Corporation completed its acquisition of all outstanding equity interests in Grace Therapeutics Inc, via a merger. Grace, based in New Jersey and organized under the laws of Delaware, was a rare and orphan disease specialty pharmaceutical company.
In connection with the share-for-share noncash transaction, Grace was merged with a new wholly owned subsidiary of Acasti and became a subsidiary of Acasti. As a result, Acasti acquired Grace’s entire therapeutic pipeline consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of various granted and pending patents in various jurisdictions worldwide. Under the terms of the acquisition, each issued and outstanding share of Grace common stock was automatically converted into the right to receive Acasti common shares equal to the equity exchange ratio set forth in the merger agreement.
Intangible assets of $
|
|
$ |
|
|
Intangible assets – in-process research and development |
|
|
|
|
GTX-104 |
|
|
|
|
GTX-102 |
|
|
|
|
GTX-101 |
|
|
|
|
Total |
|
|
|
|
5. Short-term investments
The Corporation holds various marketable securities, with maturities greater than 3 months at the time of purchase, as follows:
|
|
June 30, |
|
|
March 31, |
|
||
|
|
$ |
|
|
$ |
|
||
Term deposits issued in US currency earning interest at |
|
|
|
|
|
|
||
Term deposits issued in CAD currency earning interest at ranges between |
|
|
|
|
|
|
||
Total short-term investments |
|
|
|
|
|
|
||
14
6. Assets held for sale
During the period, the Corporation determined to actively market for sale Other assets and Production equipment and has met the criteria for classification of assets held for sale:
|
|
June 30, |
|
|
March 31, |
|
||
|
|
|
|
|
Reclassed as explained below |
|
||
|
|
$ |
|
|
$ |
|
||
Other assets (a) |
|
|
|
|
|
|
||
Production equipment (b) |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
a. Other assets
Other assets represent krill oil (“RKO”) held by the Corporation that was expected to be used in commercial inventory scale up related to the development and commercialization of the CaPre drug candidate. Given that the development of CaPre will no longer be pursued by Acasti, the Corporation is expected to sell this reserve. The other asset is being recorded at the fair value less cost to sell. Management’s estimate of the fair value of the RKO less cost to sell is based primarily on estimated market prices obtained from an appraiser specializing in the krill oil market. These projections are based on Level 3 inputs of the fair value hierarchy and reflect management’s best estimate of market participants’ pricing of the assets as well as the general condition of the asset.
b. Production equipment
June 30, 2022 |
|
Cost, net of |
|
|
Accumulated |
|
|
Net book |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Production equipment |
|
|
|
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
( |
) |
|
|
|
||
The announcement of the discontinuation of the CaPre program resulted in an impairment trigger for the laboratory and production equipment. The impairment loss is based on management’s estimate of the fair value of the equipment less cost to sell, which is based primarily on estimated market prices obtained from brokers specialized in selling used equipment. These projections are based on Level 3 inputs of the fair value hierarchy and reflect the Corporation’s best estimate of market participants’ pricing of the assets as well as the general condition of the assets.
During the three months ended June 30, 2022, the Corporation reclassed the following assets from assets held for sale as they no longer met the criteria of such classification.
|
|
Cost, net of |
|
|
Accumulated |
|
|
Net |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Furniture and office equipment |
|
|
|
|
|
( |
) |
|
|
|
||
Computer equipment |
|
|
|
|
|
( |
) |
|
|
|
||
Laboratory equipment |
|
|
|
|
|
( |
) |
|
|
|
||
|
|
|
|
|
|
( |
) |
|
|
|
||
Furthermore, depreciation expense of $
7. Capital and other components of equity
On February 14, 2019, the Corporation entered into an ATM sales agreement with B. Riley FBR, Inc. (“B. Riley”) pursuant to which common shares may be sold from time to time for aggregate gross proceeds of up to $
On June 29, 2020, the Corporation entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend the existing ATM program. Under the terms of the Sales Agreement, which has a three-year term, the Corporation may issue and sell from time-to-time common shares having an aggregate offering price of up to $
15
a commission rate equal to
On November 10, 2021, the Corporation filed a prospectus supplement relating to its at-the-market program with B. Riley, Oppenheimer& Co. Inc. and H.C. Wainwright & Co., LLC acting as agents. Under the terms of the ATM Sales Agreement and the prospectus supplement, the Corporation may issue and sell from time-to-time common shares having an aggregate offering price of up to $
During the three months ended June 30, 2022
The outstanding warrants of the Corporation are composed of the following as at June 30, 2022, and March 31, 2022:
|
|
June 30, 2022 |
|
|
March 31, 2022 |
|
||||||||||
|
|
Number |
|
|
Amount |
|
|
Number |
|
|
Amount |
|
||||
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Liability |
|
|
|
|
|
|
|
|
|
|
|
|
||||
May 2018 Canadian public offering warrants (i) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
December 2017 U.S. public offering warrants (ii) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
||||
December 2017 US public offering broker warrants (iii) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
(i)
(ii)
(iii)
8. Government assistance
Government assistance is comprised of a government grant from the Canadian federal government and research and development investment tax credits receivable from the Québec provincial government, which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. For the three months ended June 30, 2022 and 2021, the Corporation recorded $
9. Net financial income
|
|
Three Months ended |
|
|||||
|
|
June 30, |
|
|
June 30, |
|
||
|
|
$ |
|
|
$ |
|
||
Foreign exchange gain (loss) |
|
|
( |
) |
|
|
( |
) |
Change in fair value of warrant liabilities |
|
|
|
|
|
|
||
Interest income and bank charges |
|
|
|
|
|
|
||
Other income |
|
|
|
|
|
|
||
Financial income (expenses) |
|
|
( |
) |
|
|
|
|
At June 30, 2022, the Corporation has in place a stock option plan for directors, officers, employees, and consultants of the Corporation (“Stock Option Plan”). An amendment of the Stock Option Plan was approved by shareholders on August 26, 2021. The amendment provides for an increase to the existing limits for common shares reserved for issuance under the Stock Option Plan as well as certain changes to the minimum vesting period applicable to options granted to directors under the Stock Option Plan.
The Stock Option Plan continues to provide for the granting of options to purchase common shares. The exercise price of the stock options granted under this amended plan is not lower than the closing price of the common shares on the TSXV at the close of markets the day preceding the grant. The maximum number of common shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 10% of the aggregate
16
number of issued and outstanding shares of the Corporation. The terms and conditions for acquiring and exercising options are set by the Corporation’s Board of Directors, subject among others, to the following limitations: the term of the options cannot exceed years and (i) all options granted to a director will be vested evenly on a monthly basis over a period of at least twelve (12) months, and (ii) all options granted to an employee will be vested evenly on a quarterly basis over a period of at least thirty-six (36) months.
The total number of shares issued to any one consultant within any twelve-month period cannot exceed
The following table summarizes information about activities within the Stock Option Plan for the three month period ended:
|
|
June 30, 2022 |
|
|
June 30, 2021 |
|
||||||||||
|
|
Weighted average |
|
|
Number of |
|
|
Weighted average |
|
|
Number of |
|
||||
|
|
CAD$ |
|
|
|
|
|
CAD$ |
|
|
|
|
||||
Outstanding at beginning of period |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Forfeited |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
||
Expired |
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|||
Outstanding at end of period |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercisable at end of period |
|
|
|
|
|
|
|
|
|
|
|
|||||
The fair value of options granted was estimated using the Black-Scholes option pricing model, resulting in the following weighted average assumptions for the options granted:
|
Three Months ended |
|
||
|
June 30, |
|
||
|
|
$ |
|
|
Exercise price |
CAD $ |
|
|
|
Share price |
CAD $ |
|
|
|
Weighted average grant-date fair value per award |
CAD $ |
|
|
|
Volatility |
|
|
% |
|
Risk-free interest rate |
|
|
% |
|
Expected life |
|
|
|
|
Dividend |
|
|
|
|
Stock-based compensation payment transactions
The fair value of stock-based compensation transactions is measured using the Black-Scholes option pricing model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility for a duration equal to the estimated weighted average life of the instruments, life based on the average of the vesting and contractual periods for employee awards as minimal prior exercises of options in which to establish historical exercise experience; and contractual life for broker warrants), and the risk-free interest rate (based on government bonds). Service and performance conditions attached to the transactions, if any, are not taken into account in determining fair value. The expected life of the stock options is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility over a period similar to the life of the options is indicative of future trends, which may also not necessarily be the actual outcome.
Compensation expense recognized under the Stock Option Plan for the three months ended June 30, 2022 and 2021 was as follows:
|
|
Three Months ended |
|
|||||
|
|
June 30, |
|
|
June 30, |
|
||
|
|
$ |
|
|
$ |
|
||
Research and development expenses |
|
|
|
|
|
|
||
General and administrative expenses |
|
|
|
|
|
|
||
Sales and marketing expenses |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
17
11. Supplemental cash flow disclosure
(a) Changes in non-cash operating items
|
|
Three Months ended |
|
|||||
|
|
June 30, |
|
|
June 30, |
|
||
|
|
$ |
|
|
$ |
|
||
Receivables |
|
|
( |
) |
|
|
|
|
Prepaid expenses |
|
|
( |
) |
|
|
( |
) |
Trade and other payables |
|
|
|
|
|
|
||
|
|
|
( |
) |
|
|
( |
) |
12. Commitments and contingencies
Research and development contracts and contract research organizations agreements
We utilize contract manufacturing organizations, for the development and production of clinical materials and contract research organizations to perform services related to our clinical trials. Pursuant to the agreements with these contract manufacturing organizations and contract research organizations, we have either the right to terminate the agreements without penalties or under certain penalty conditions.
Supply contract
On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antartic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre for a total fixed value of $
Legal proceedings and disputes
In the ordinary course of business, the Corporation is at times subject to various legal proceedings and disputes. The Corporation assess its liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that the Corporation will incur a loss and the amount of the loss can be reasonably estimated, the Corporation records a liability in its consolidated financial statements. These legal contingencies may be adjusted to reflect any relevant developments. Where a loss is not probable or the amount of loss is not estimable, the Corporation does not accrue legal contingencies. While the outcome of legal proceedings is inherently uncertain, based on information currently available, management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on the Corporation’s financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to the Corporation’s financial position, results of operations, or cash flows. No reserves or liabilities have been accrued as at June 30, 2022.
18
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operation
This management’s discussion and analysis (“MD&A”) is presented in order to provide the reader with an overview of the financial results and changes to our balance sheet as at June 30, 2022, and for the three-month period then ended. This MD&A also explains the material variations in our results of operations, balance sheet and cash flows for the three months ended June 30, 2022 and 2021.
Market data, and certain industry data and forecasts included in this MD&A were obtained from internal corporation surveys and market research and those conducted by third parties hired by us, publicly available information, reports of governmental agencies and industry publications, and independent third-party surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of that information is not guaranteed. We have not independently verified any of the data from third-party sources or the underlying economic assumptions they have made. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s or contracted third parties’ knowledge of our industry, have not been independently verified. Our estimates involve risks and uncertainties, including assumptions that may prove not to be accurate, and these estimates and certain industry data are subject to change based on various factors, including those discussed in this quarterly report and in our most recently filed annual report on Form 10-K.
This MD&A, approved by the Board of Directors on August 11, 2022, should be read in conjunction with our unaudited condensed consolidated interim financial statements for the three months ended June 30, 2022 and 2021 included elsewhere in this quarterly report. Our interim financial statements were prepared in accordance with U.S. GAAP.
All amounts appearing in this MD&A for the period-by-period discussions are in thousands of U.S. dollars, except share and per share amounts or unless otherwise indicated.
Business Overview
On August 27, 2021, we completed our acquisition of Grace via a merger following the approval of Acasti’s shareholders and Grace’s stockholders. Following completion of the merger, Grace became a wholly owned subsidiary of Acasti and was renamed Acasti Pharma U.S. Inc.
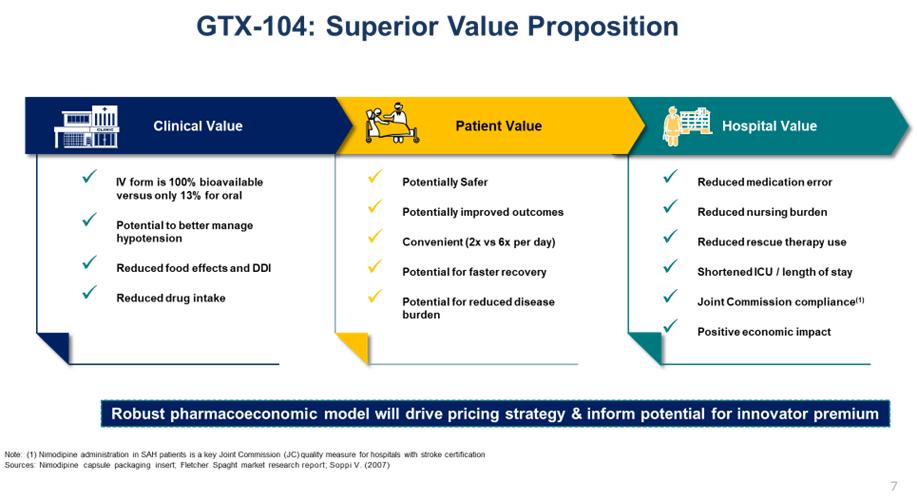
The successful completion of the merger positions Acasti to build a premier, late-stage specialty pharmaceutical company focused on developing and commercializing products for rare and orphan diseases that have the potential to improve clinical outcomes by using the Company’s novel drug delivery technologies. We seek to apply new proprietary formulations to approved and marketed pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, and more convenient drug delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by Acasti for further development may be already approved in a target indication or could be repurposed for use in new indications.
The existing well understood efficacy and safety profiles of these marketed compounds provides the opportunity for us to utilize the Section 505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act (the “FFDCA”) for the development of our reformulated versions of these drugs, and therefore may provide a potentially shorter path to regulatory approval. Under Section 505(b)(2), if sufficient support of a product’s safety and efficacy either through previous FDA experience or sufficiently within the scientific literature can be established, it may eliminate the need to conduct some of the early studies that new drug candidates might otherwise require.
In connection with the merger, we acquired Grace’s entire therapeutic pipeline, which has the potential to address critical unmet medical needs for the treatment of rare and orphan diseases. The pipeline consists of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio of more than 40 granted and pending patents in various jurisdictions worldwide. These drug candidates aim to improve clinical outcomes by applying proprietary formulation and drug delivery technologies to existing pharmaceutical compounds to achieve improvements over the current standard of care, or to provide treatment for diseases with no currently approved therapies.
Rare disorders represent an attractive area for drug development, and there remains an opportunity for Acasti to utilize already approved drugs that have established safety profiles and clinical experience to potentially address significant unmet medical needs. A key advantage of pursuing therapies for rare disorders is the potential to receive orphan drug designation (“ODD”) from the FDA. ODD provides for seven years of marketing exclusivity in the United States post-launch, provided certain conditions are met, and the potential for faster regulatory review. Rare diseases also allow for more manageably scaled clinical trials and provide market opportunities that may require a smaller, more targeted commercial infrastructure.
The specific diseases targeted for drug development by Acasti are well understood although these patient populations may remain poorly served by available therapies or in some cases approved therapies do not yet exist. We aim to effectively treat debilitating symptoms that result from these underlying diseases.
Our three most advanced programs are:
19
Our management team possesses significant experience in drug formulation and drug delivery research and development, clinical and pharmaceutical development and manufacturing, regulatory affairs, and business development, as well as being well-versed in late-stage drug development and commercialization. The Acasti team has been collectively involved in the development and approval of numerous successfully marketed drugs, including TORADOL, NAPROSYN, ANDROGEL, SUBSYS, MARINOL, KEPPRA XR, CLARITIN®, EUFLEX®, EFFEXOR®, SONATA®, ATIVAN®, RD-HEPARIN®, RAPAMUNE®, ETODOLAC, ARICEPT®, CARDIZEM®, DEFLAZACORT®, AND MACIMORELIN®.
The table below summarizes planned key fiscal 2023 milestones for our three clinical drug candidates:
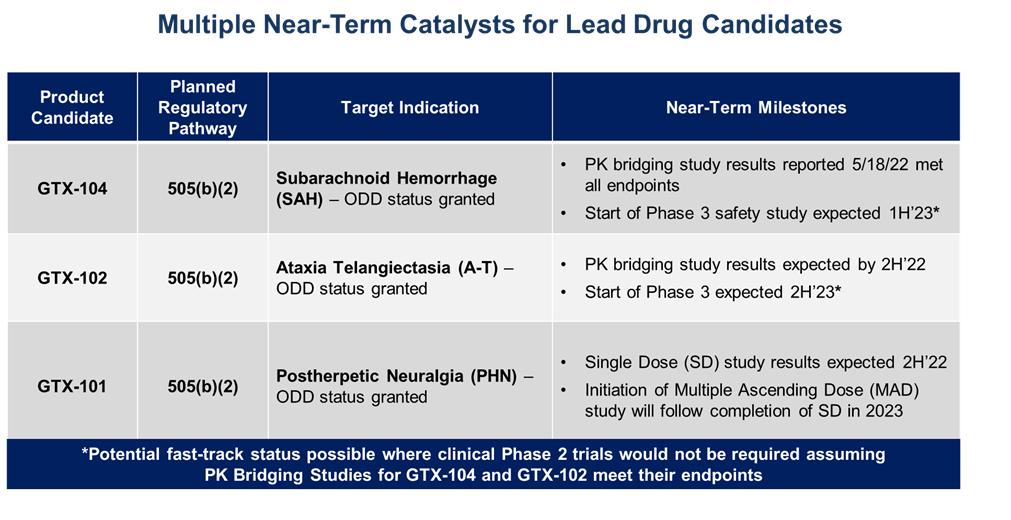
GTX-104 Overview
Nimodipine was granted FDA approval in 1988, and is the only drug approved to improve neurological outcomes in SAH. It is only available in the United States as a generic oral capsule and as a branded oral liquid solution called NYMALIZE, which is manufactured and sold by Arbor Pharmaceuticals (acquired in September 2021 by Azurity Pharmaceuticals). Nimodipine has poor water solubility and high permeability characteristics as a result of its high lipophilicity. Additionally, orally administered nimodipine has dose-limiting side-effects such as hypotension, poor absorption and low bioavailability resulting from high first-pass metabolism, and a narrow administration window as food effects lower bioavailability significantly. Due to these issues, blood levels of orally administered nimodipine can be highly variable, making it difficult to manage blood pressure in SAH patients. Nimodipine capsules are also difficult to administer, particularly to unconscious patients or those with impaired ability to swallow. Concomitant use with CYP3A inhibitors is contraindicated (NIMODIPINE Capsule PI).
NIMOTOP is an injectable form of nimodipine that is manufactured by Bayer Healthcare. It is approved in Europe and in other regulated markets (but not in the United States), but it has limited utility for SAH patients because of its high organic solvent content, namely 23.7% ethanol and 17% polyethylene glycol 400 (NIMOTOP SmPC).
GTX-104 is a clinical stage, novel formulation of nimodipine for IV infusion in SAH patients. It uses surfactant micelles as the drug carrier to solubilize nimodipine. This unique nimodipine injectable formulation is composed of a nimodipine base, an effective amount of polysorbate 80, a non-ionic hydrophilic surfactant, and a pharmaceutically acceptable carrier for injection. GTX-104 is an aqueous solution substantially free of organic solvents, such that the nimodipine is contained in a concentrated injection solution, suspension, emulsion or complex as a micelle, a colloidal particle or an inclusion complex, and the formulation is stable and clear.
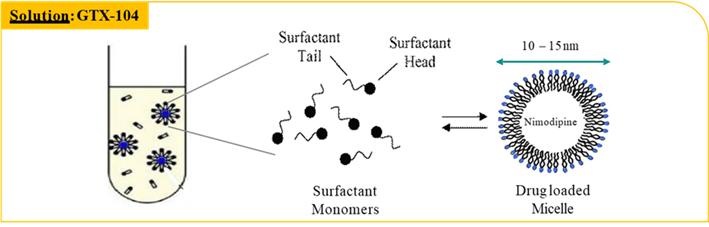
Key Benefits: Novel nanoparticle technology facilitates aqueous formulation of insoluble nimodipine and enables a safe, standard peripheral IV infusion:
20
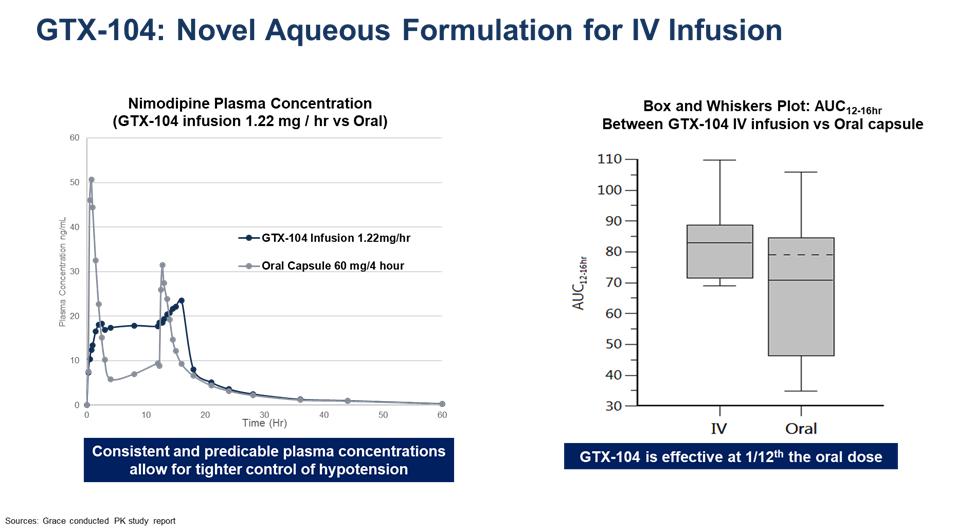
GTX-104 could provide a more convenient mode of administration as compared to generic nimodipine capsules or NYMALIZE GTX-104 is peripherally infused every four hours as compared to oral administration via a nasogastric tube in unconscious patients every two to four hours for both NYMALIZE solution and nimodipine capsules. Therefore, GTX-104 could be considered as a major contribution to patient care by potentially reducing the dosing frequency, and the associated nursing burden. More convenient and less frequent dosing can also reduce the risk of medication errors. In addition, two PK studies conducted with GTX-104 have shown that it has the potential to provide improved bioavailability and lower intra-subject variability compared to oral administration (see chart below). Because of its IV formulation, we also expect it to reduce certain drug-drug interactions and food effects.
Despite the positive impact it has on recovery, physicians often must discontinue their patients on oral nimodipine, primarily as a result of hypotensive episodes that cannot be controlled by titrating the oral form of drug. Such discontinuation could potentially be avoided by administering GTX-104, which because of its IV administration, may obviate the complexity that results from the need for careful attention to the timing of nimodipine administration at least one hour before or two hours after a meal. Administration of GTX-104 via a peripheral vein is often much more comfortable for the patients compared to administration by central venous access, which can often be a difficult and invasive procedure. Also, unconscious patients will likely receive more consistent concentrations of nimodipine when delivered by the IV route as compared to oral gavage or a nasogastric tube. More consistent dosing is expected to result in a reduction of vasospasm and a better, more consistent management of hypotension. As summarized in the table below, we anticipate reduced use of rescue therapies, such as vasopressors, and expensive hospital resources, such as the angiography suite, are possible by more effectively managing blood pressure with GTX-104. Reduced incidences of vasospasm could result in shorter length of stay and better outcomes.
21
About Subarachnoid Hemorrhage (SAH)
SAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is rupture of an aneurysm. The result is a relatively uncommon type of stroke that accounts for about 5% of all strokes and has an incidence of six per 100,000 person years (Becske, 2018).
In contrast to more common types of stroke in elderly individuals, SAH often occurs at a relatively young age, with approximately half the affected patients younger than 60 years old (Becske, 2018). Particularly devastating for patients younger than 45, around 10% to 15% of aneurysmal SAH (“aSAH”) patients die before reaching the hospital (Rinkel, 2016), and those who survive the initial hours post hemorrhage are admitted or transferred to tertiary care centers with high risk of complications, including rebleeding and delayed cerebral ischemia (“DCI”). Systemic manifestations affecting cardiovascular, pulmonary, and renal function are common and often complicate management of DCI. Approximately 70% of aSAH patients experience death or a permanent dependence on family members, and half die within one month after the hemorrhage. Of those who survive the initial month, half remain permanently dependent on a caregiver to maintain daily living (Becske, 2018).
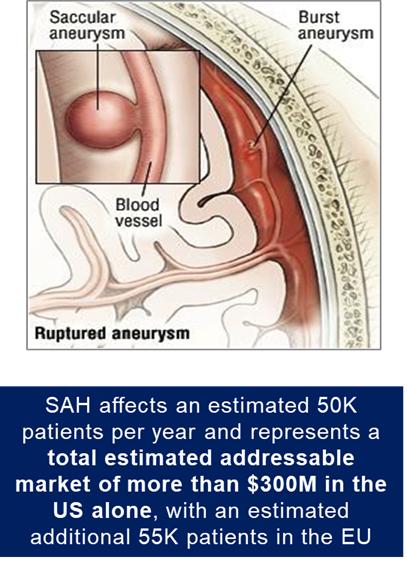
We estimate that approximately 53,596 individuals experience aSAH each year. The total addressable market for SAH is approximately $300 million in the U.S., and an estimated 50,000 patients in the European Union based on annual inpatient admissions and the average length-of-stay.
22
GTX-104 Near Term Milestones: Conduct Phase 3 Safety Study
In September 2021, we initiated our pivotal PK bridging study to evaluate the relative bioavailability of GTX-104 compared to currently marketed oral nimodipine capsules in approximately 50 healthy subjects. The PK study was the next required step in our proposed 505(b)(2) regulatory pathway for GTX-104.
Final results from this pivotal PK study were reported on May 18, 2022, and showed that the bioavailability of IV GTX-104 compared favorably with the oral formulation of nimodipine in all subjects, and no serious adverse events were observed for GTX-104.
All three endpoints indicated that statistically there was no difference in exposures between IV GTX-104 and oral nimodipine over the defined time periods for both maximum exposure and total exposure. Plasma concentrations obtained following IV administration showed significantly less variability between subjects as compared to oral administration of capsules, since IV administration is not as sensitive to some of the physiological processes that affect oral administration, such as taking the drug with and without meals, variable gastrointestinal transit time, variable drug uptake from the gastrointestinal tract into the systemic circulation, and variable hepatic blood flow and hepatic first pass metabolism. Previous studies have shown these processes significantly affect the oral bioavailability of nimodipine, and therefore cause oral administration to be prone to larger within and between-subject variability.
The bioavailability of oral nimodipine capsules observed was only 8% compared to 100% for IV GTX-104. Consequently, about one-twelfth the amount of nimodipine is delivered with GTX-104 to achieve the same blood levels as with the oral capsules.
No serious adverse events and no adverse events leading to withdrawal were reported during the study.
Next Step – Initiate Phase 3 Safety Study for GTX-104
We plan to submit the final PK bridging study report to the FDA in calendar Q3, 2022. We also intend to request a Type C meeting with the FDA to get the agency’s guidance on our proposed phase 3 study design. We expect to receive that guidance by the end of 2022 or early in the first calendar quarter of 2023. This should allow us to initiate the Phase 3 Safety Study and enroll the first patient in the first half of 2023. The study is expected to take about 18 months to complete, and we expect this safety study to be the final clinical step required to seek approval under the 505(b)(2) regulatory pathway. Before submitting a New Drug Application to the FDA, Acasti plans to hold a pre-NDA meeting to enhance the likelihood of market approval.
GTX-102 Overview
GTX-102 is a novel, concentrated oral-mucosal spray of betamethasone intended to improve neurological symptoms of Ataxia Telangiectasia (“A-T”) for which there are currently no FDA-approved therapies. GTX-102 is a stable, concentrated oral spray formulation comprised of the glucocorticoid betamethasone, that together with other excipients can be sprayed conveniently over the tongue of the A-T patient.
About Ataxia Telangiectasia
A-T is a rare genetic progressive autosomal recessive neurodegenerative disorder that affects children, with the hallmark symptoms of cerebellar ataxia and other motor dysfunction, and dilated blood vessels (telangiectasia) that occur in the sclera of the eyes. A-T is caused by mutations in the ataxia telangiectasia gene, which is responsible for modulating cellular response to stress, including breaks in the double strands of DNA.
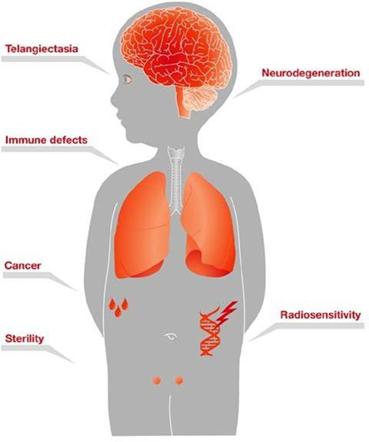
Children with A-T begin to experience balance and coordination problems when they begin to walk (toddler age), and ultimately become wheelchair-bound in their second decade of life. In pre-adolescence (between ages 5 and 8), patients experience oculomotor apraxia, dysarthria, and dysphagia. They also often develop compromised immune systems and are at increased risk of developing respiratory tract infections and cancer (typically lymphomas and leukemia) (U.S. National Cancer Institute A-T, 2015).
23
A-T is diagnosed through a combination of clinical assessment (especially neurologic and oculomotor deficits), laboratory analysis, and genetic testing. There is no known treatment to slow disease progression, and treatments that are used are strictly aimed at controlling the symptoms (e.g., physical, occupational or speech therapy for neurologic issues), or conditions secondary to the disease (e.g., antibiotics for lung infections, chemotherapy for cancer, etc.) (U.S. National Cancer Institute A-T, 2015). There are no FDA-approved therapeutic options currently available. Patients typically die by age 25 from complications of lung disease or cancer. According to a third-party report commissioned by Acasti Pharma US, A-T affects approximately 4,300 patients per year in the United States and has a potential total addressable market of $150 million, based on the number of treatable patients in the United States.
GTX-102 - R&D and Clinical Studies to Date
In a multicenter, double-blind, randomized, placebo-controlled crossover trial conducted in Italy, Zannolli et al. studied the effect of an oral liquid solution of betamethasone on the reduction of ataxia symptoms in 13 children (between ages 2 to 8 years) with A-T. The primary outcome measure was the reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”).
In the trial, oral liquid betamethasone reduced the ICARS total score by a median of 13 points in the intent-to-treat (“ITT”) population and 16 points in the per-protocol (“PP”) population (the median percent decreases of ataxia symptoms of 28% and 31%, respectively). Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require medical intervention. Clinical study results in A-T patients administered oral betamethasone indicated that betamethasone significantly reduced ICARS total score relative to placebo (P = 0.01). The median ICARS change score (change in score with betamethasone minus change in score with placebo) was -13 points (95% confidence interval for the difference in medians was -19 to -5.5 points).
Based on the Zannolli data, we believe GTX-102 concentrated oral spray has the potential to provide clinical benefits in decreasing A-T symptoms, including assessments of posture and gait disturbance and kinetic, speech and oculomotor functions. In addition, GTX-102 may ease drug administration for patients experiencing A-T given its application of 1-3x/day of 140µL of concentrated betamethasone liquid spray onto the tongue using a more convenient metered dose spray, as these A-T patients typically have difficulty swallowing (lefton-greif 2000).
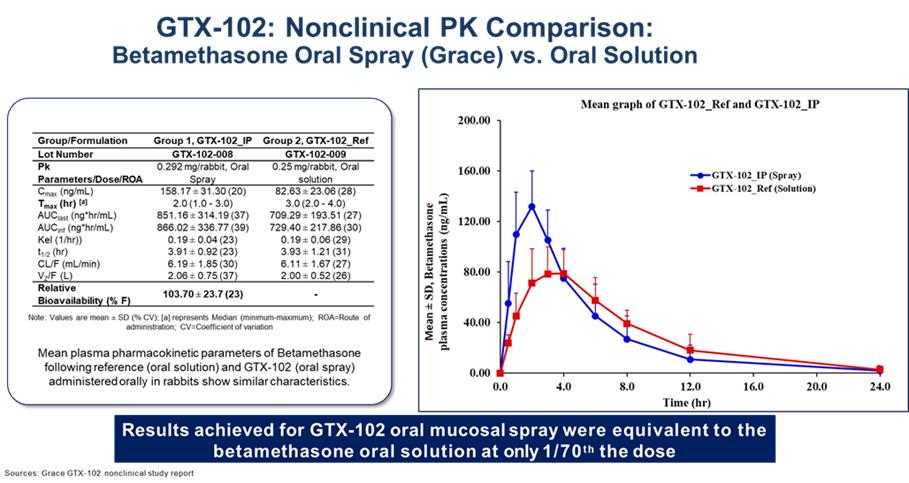
GTX-102 PK Data to Date:
GTX-102 administered as a concentrated oral spray achieves similar blood levels at only 1/70th the volume of an oral solution of betamethasone. This is important for A-T patients who have difficulties swallowing large volumes of liquids, and it could help to reduce the side effects common with chronic use of a glucocorticosteroid drug.
GTX-102 Near-Term Milestones: Conduct PK Bridging and Confirmatory Phase 3 Clinical Trials
Acasti Pharma US has licensed the data from the multicenter, double-blinded, randomized, placebo-controlled crossover trial from Azienda Ospedaliera Universitaria Senese, Siena, Italy, where Dr. Zannolli et. al. studied the effect of oral liquid solution of betamethasone to reduce ataxia symptoms in patients with A-T. Note that this oral liquid solution is not approved in the United States, and therefore is not available for clinical use. Betamethasone is only available in the United States as an injectable or as a topical cream. However, this license gives Acasti Pharma US the right to reference the study’s data in its NDA filing. On November 12, 2015, Acasti Pharma US submitted the data from the Zannolli study to the FDA’s Division of Neurology at a pre-Investigational New Drug (“IND”) meeting and received guidance from the agency on the regulatory requirements to seek approval.
We plan to initiate a PK bridging study of our proprietary concentrated oral spray as compared to the oral liquid solution of betamethasone used in the Zannolli study and against the injectable form of betamethasone that is approved in the U.S. in the third calendar quarter of 2022. We expect to report the results of this study before the end of 2022. Assuming the PK bridging study meets its primary endpoint, we plan to conduct a confirmatory Phase 3 safety and efficacy trial in A-T patients, and plan to seek guidance from the FDA on the study design at a Type B meeting. The Phase 3 study is expected to be initiated in the second half of 2023. If both studies meet their primary endpoints, a Pre-NDA meeting and an NDA filing under Section 505(b)(2) would follow.
24
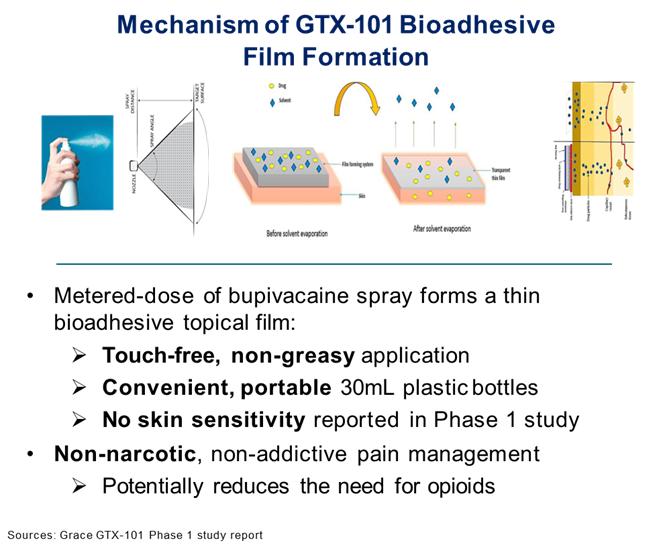
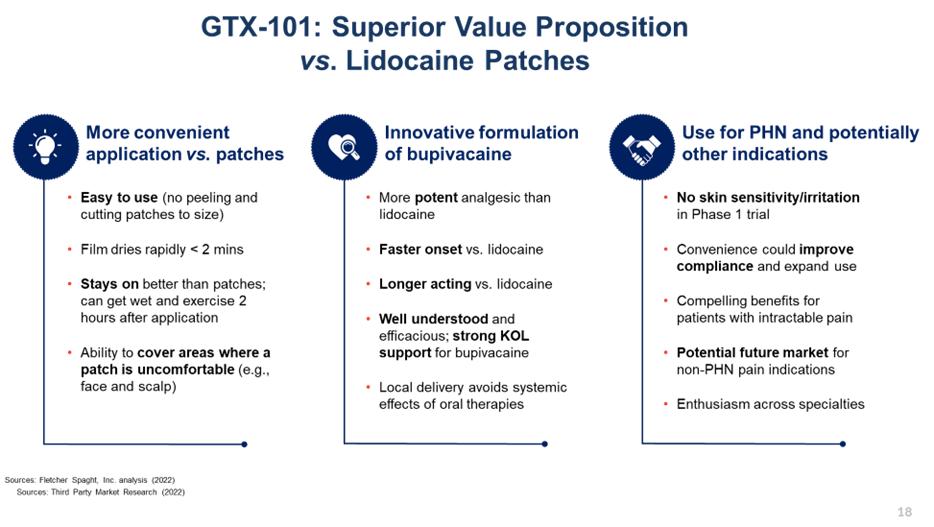
GTX-101 Overview
GTX-101 is a non-narcotic, topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (“PHN”). GTX-101’s metered-dose of bupivacaine spray forms a thin bio-adhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches, we believe that the biphasic delivery mechanism of GTX-101 has the potential for rapid onset and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 study.
About Postherpetic Neuralgia (PHN)
25
PHN is neuropathic pain due to damage caused by the varicella zoster virus (“VZV”). Infection with the VZV causes two distinct clinical conditions. Primary VZV infection causes varicella (i.e., chickenpox), a contagious rash illness that typically occurs among young children. Secondary VZV can reactivate clinically, decades after initial infection, to cause herpes zoster (“HZ”), otherwise known as shingles. Acute HZ arises when dormant virus particles, persisting within an affected sensory ganglion from the earlier, primary infection with VZV become reactivated when cellular immunity to varicella decreases. Viral particles replicate and may spread to the dorsal root, into the dorsal horn of the spinal cord, and through peripheral sensory nerve fibers down to the level of the skin. Viral particles also may circulate in the blood. This reactivation is accompanied by inflammation of the skin, immune response, hemorrhage, and destruction of peripheral and central neurons and their fibers. Following such neural degeneration, distinct types of pathophysiological mechanisms involving both the central and peripheral nervous systems may give rise to the severe nerve pain associated with PHN.
While the rash associated with HZ typically heals within two to four weeks, the pain may persist for months or even years, and this PHN manifestation is the most common and debilitating complication of HZ. There is currently no consensus definition for PHN, but it has been suggested by the Centers for Disease Control and Prevention (“CDC”) that PHN is best defined as pain lasting at least three months after resolution of the rash.
PHN is associated with significant loss of function and reduced quality of life, particularly in the elderly. It has a detrimental effect on all aspects of a patients’ quality of life. The nature of PHN pain varies from mild to excruciating in severity, constant, intermittent, or triggered by trivial stimuli. Approximately half of patients with PHN describe their pain as “horrible” or “excruciating,” ranging in duration from a few minutes to constant on a daily or almost daily basis (Katz, 2004). The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the quality of life and leading to social withdrawal and depression. PHN is the number-one cause of intractable, debilitating pain in the elderly, and has been cited as the leading cause of suicide in chronic pain patients over the age of 70 (Hess, 1990).
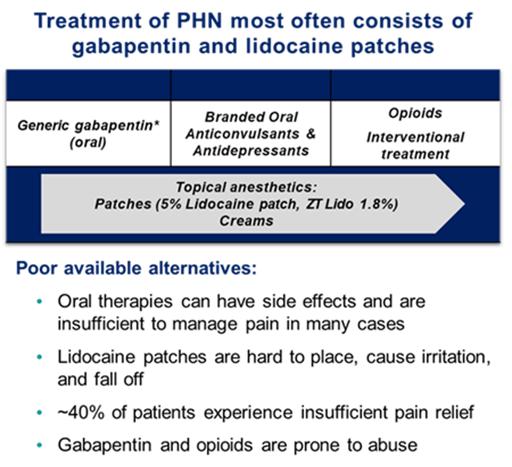
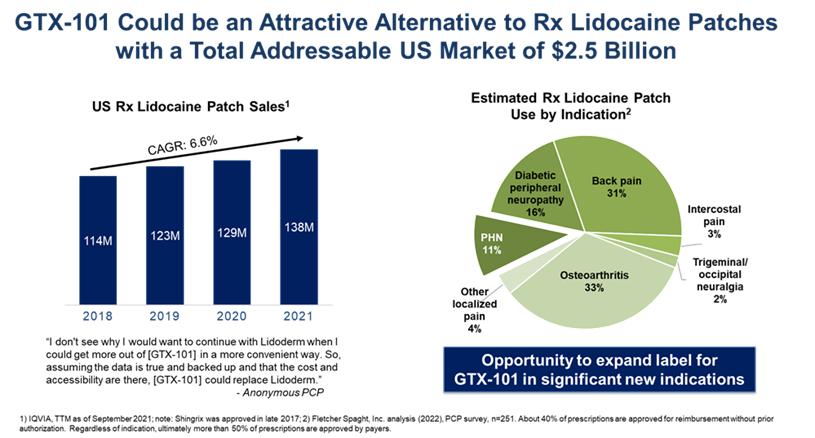
Current treatment of PHN most often consists of oral gabapentin (first line) and prescription lidocaine patches or antidepressants (second line), and refractory cases may be prescribed opioids to address persistent pain. Gabapentin and opioid abuse have continued to proliferate, and lidocaine patches are suboptimal for many reasons. An independent third party market research firm commissioned by Acasti interviewed more than 250 physicians who regularly treat PHN patients, and found that approximately 40% of patients using lidocaine patches experience insufficient pain relief. Lidocaine patches are difficult to use, fall off, and look unsightly with possible skin sensitivity and irritation. Additionally, it can take up to two weeks for an optimal analgesic effect to be achieved. Prescription lidocaine patches are only approved for PHN, and the market is currently made up of both branded and generic offerings. It is estimated that PHN affects approximately 120,000 patients per year in the United States. According to the third-party report commissioned by Acasti, the total addressable market for GTX-101 could be as large as $2.5 billion, consisting of approximately $200 million for PHN pain and $2.3 billion for non-PHN pain.
26
GTX-101 R&D History and Clinical Studies Completed to Date
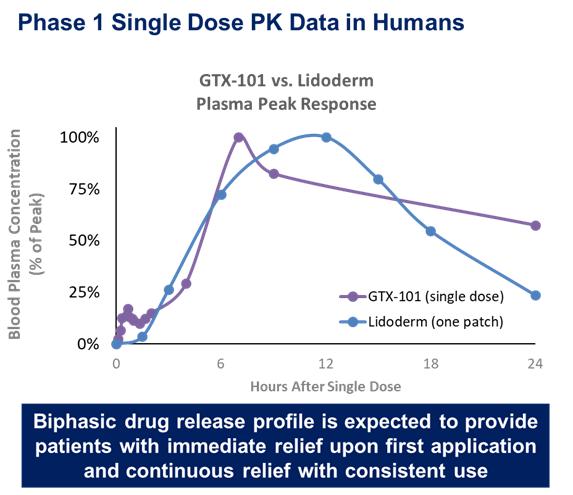
To date, Acasti Pharma US has conducted three Phase I studies in healthy volunteers to assess the PK, safety and tolerability of GTX-101 and to determine the plasma levels of bupivacaine HCl administered as a single dose in various concentrations, namely 30 mg (three sprays), 50 mg (five sprays), 70 mg (seven sprays) or 100 mg (ten sprays).
These studies confirmed that bupivacaine delivered as a topical spray (GTX-101) is well absorbed through the skin, as demonstrated in the graph below, while very little is absorbed systemically.
In all three studies, the administration of GTX-101 to healthy volunteers was safe and well tolerated. In addition, no evidence of skin irritation was observed at the application site following the spray administrations.
GTX-101 Near-Term Milestones: Conduct Dose Ranging Phase 1 Clinical Trials of GTX-101
We believe that the PHN pain market will continue to grow, and non-opioid products like GTX-101 that can relieve PHN pain more quickly and in a sustained manner by means of a more efficient delivery system, will be an attractive therapy option for patients and physicians. GTX-101 is administered by spraying our proprietary bupivacaine formulation over the affected area, which we believe has the potential to provide several advantages over currently marketed products such as the lidocaine patch, including faster onset of action, sustained pain relief, possibly lower dosing requirements and improved dosing convenience, all which could lead to increased patient satisfaction and compliance.
GTX-101: The data from the single dose Phase 1 clinical trial for topical bupivacaine spray was submitted to the FDA’s Division of Anesthesiology and feedback was received at the pre-IND meeting on April 18, 2018, that informed the design of preclinical toxicology studies and a clinical and regulatory
27
pathway to approval under section 505(b)(2). We completed a minipig skin sensitivity study in the second calendar quarter of 2022, and we initiated a single dose PK study in healthy human volunteers on July 26th, 2022. This single dose PK study is expected to report out before the end of 2022 and will be followed by the multiple ascending dose study in 2023. Results from the these non-clinical and clinical studies are required before the initiation of our Phase 2 program in PHN patients.
Overall Commercialization Strategy
We plan to retain our worldwide commercialization rights for some of our key drug candidates, while for other drug candidates we might consider collaboration opportunities to maximize market penetration and returns. If we receive regulatory approval, we expect to build a small and focused commercial organization in the United States to market and sell GTX-104 and GTX-102. We believe the patient populations and medical specialists for these indications are sufficiently concentrated to allow us to cost-effectively promote these drug products following approval for commercial sale. Given that GTX-101 will be targeted to a larger primary care and pain specialist market, if GTX-101 receives regulatory approval, it is likely we will seek commercial partnerships to fully exploit the market potential of this drug product.
As product candidates advance through the pipeline, our commercial plans may change. Clinical data, the size of the development programs, the size of the target market, the size of a commercial infrastructure and manufacturing needs may all have influence on U.S., European Union, and rest-of-world strategies.
Recent Developments
Annual Stock Option Grants
On June 22, 2022, we announced the annual grant of stock options to our employees and executives. An aggregate of 1,212,500 options to purchase common shares of the Company were granted under our stock option plan. The stock options were granted by the board of directors as part of the annual performance review in accordance with our Long-Term Incentive Program. Subject to the terms and conditions of the Stock Option Plan, each stock option will entitle the holder to purchase one common share of Acasti at an exercise price of CDN 1.15 and will expire after ten years. In accordance with the Stock Option Plan, options granted to executives and employees will vest in equal quarterly instalments over a period of 36 months.
Initiation of Pharmacokinetic Study for GTX-101
On July 27, 2022, we announced the initiation of our planned pharmacokinetic (PK) bridging study to evaluate the relative bioavailability of GTX-101, our drug candidate for the treatment of Postherpetic Neuralgia compared to the reference listed drug bupivacaine in 48 healthy subjects.
Nasdaq Letter
On July 27, 2022, we received written notification from the Nasdaq Listing Qualifications Department ("Nasdaq") for failing to maintain a minimum bid price of $1.00 per common share for the last 30 consecutive business days, as required by Nasdaq Listing Rule 5550(a)(2) - bid price (the "Minimum Bid Price Rule"). The Nasdaq notification has no immediate effect on the listing of the our common shares, and we have 180 calendar days, or until January 23, 2023, to regain compliance. If at any time over this period the bid price of our common shares closes at $1.00 per share or more for at least a minimum of ten (10) consecutive business days, Nasdaq will provide written confirmation of compliance and the matter will be closed.
If the we do not regain compliance within the initial 180-day period, but otherwise meets the continued listing requirements for market value of publicly-held shares and all other initial listing standards for the Nasdaq Listing Rule 5505 - Capital Market criteria, except for the Minimum Bid Price Rule, we may be eligible for an additional 180 calendar days to regain compliance. If we are not granted additional time, then our common shares will be subject to delisting, at which time we may appeal the delisting determination to a Nasdaq Hearings Panel.
We intend to monitor the closing bid price of its common shares and, if necessary, evaluate all available options to resolve the deficiency and regain compliance with the Minimum Bid Price Rule.
COVID-19 Update
To date, the ongoing COVID-19 pandemic has not caused significant disruptions to our business operations and research and development activities.
The extent to which the COVID-19 pandemic impacts our business and prospects and the timing and completion of future clinical trials for our new drug candidates will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the COVID-19 pandemic and the actions to contain the COVID-19 pandemic or treat its impact, among others.
Basis of Presentation of the Financial Statements
Our condensed consolidated interim financial statements, which include the accounts of our wholly owned subsidiaries, Acasti Innovations AG and Acasti Pharma U.S., have been prepared in accordance with U.S. GAAP and the rules and regulations of the SEC related to quarterly reports filed on Form 10-Q. All intercompany transactions and balances are eliminated on consolidation.
Our assets as at June 30, 2022, include cash and cash equivalents and short-term investments totaling $38.4 million and intangible assets and goodwill totaling $82.8 million. Our current liabilities total $3.7 million as at June 30, 2022 and are comprised primarily of amounts due to or accrued for creditors.
Comparative Financial Information for the Three Months ended June 30, 2022 and 2021
28
|
|
Three Months ended |
|
|||||||||
|
|
June 30, |
|
|
June 30, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Net loss |
|
|
(4,524 |
) |
|
|
(3,118 |
) |
|
|
1,406 |
|
Basic and diluted gain (loss) per share |
|
|
(0.10 |
) |
|
|
(0.12 |
) |
|
|
(0.02 |
) |
Total assets |
|
|
124,931 |
|
|
|
60,453 |
|
|
|
64,478 |
|
Working capital1 |
|
|
37,541 |
|
|
|
58,042 |
|
|
|
(20,501 |
) |
Total non-current financial liabilities |
|
|
174 |
|
|
|
4,651 |
|
|
|
(4,477 |
) |
Total shareholders’ equity |
|
|
104,403 |
|
|
|
53,457 |
|
|
|
50,946 |
|
1 Working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by U.S. GAAP requirements, the results may not be comparable to similar measurements presented by other public companies.
Results of Operations for the Three Months ended June 30, 2022 and 2021
|
|
Three Months ended |
|
|||||||||
|
|
June 30, |
|
|
June 30, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|||
Operating expenses |
|
|
|
|
|
|
|
|
|
|||
Research and development expenses, net of government assistance |
|
|
2,590 |
|
|
|
469 |
|
|
|
2,121 |
|
General and administrative expenses |
|
|
1,919 |
|
|
|
2,676 |
|
|
|
(757 |
) |
Sales and marketing expenses |
|
|
221 |
|
|
|
— |
|
|
|
221 |
|
Loss from operating activities |
|
|
(4,730 |
) |
|
|
(3,145 |
) |
|
|
1,585 |
|
|
|
|
|
|
|
|
|
|
|
|||
Financial Income (expense) |
|
|
(36 |
) |
|
|
27 |
|
|
|
(63 |
) |
Income tax recovery |
|
|
242 |
|
|
|
— |
|
|
|
242 |
|
Net loss |
|
|
(4,524 |
) |
|
|
(3,118 |
) |
|
|
1,406 |
|
Net Loss
The net loss of $4,524 or $0.10 per share for the three months ended June 30, 2022, increased by $1,406 from the net loss of $3,118 or $0.12 per share for the three months ended June 30, 2022.
Research and development expenses
Research and development expenses consist primarily of:
We record research and development expenses as incurred.
Our research and development during the three months ended June 30, 2022 was focused primarily on our clinical development programs GTX-104, GTX-102, and GTX-101 drug candidates, which were acquired in the Grace merger on August 27, 2021. Research and development expenses during the three months ended June 30, 2021, related to the completion of our TRILOGY Phase 3 clinical program for our former drug candidate, CaPre.
The following table summarizes our research and development expenses:
29
Research and development expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Three Months Ended |
|
|||||||||
|
|
June 30, |
|
|
June 30, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Third-party contract research expenses: |
|
|
|
|
|
|
|
|
|
|||
Clinical development programs: |
|
|
|
|
|
|
|
|
|
|||
GTX-104 |
|
|
323 |
|
|
|
— |
|
|
|
323 |
|
GTX-102 |
|
|
567 |
|
|
|
— |
|
|
|
567 |
|
GTX-101 |
|
|
637 |
|
|
|
— |
|
|
|
637 |
|
Other third-party contract research expenses |
|
|
154 |
|
|
|
76 |
|
|
|
78 |
|
Professional fees |
|
|
243 |
|
|
|
16 |
|
|
|
227 |
|
Other research and development costs |
|
|
88 |
|
|
|
33 |
|
|
|
55 |
|
Government grants & tax credits |
|
|
(190 |
) |
|
|
— |
|
|
|
(190 |
) |
Total third-party research and development expenses1 |
|
|
1,822 |
|
|
|
125 |
|
|
|
1,697 |
|
Salaries and benefits |
|
|
489 |
|
|
|
294 |
|
|
|
195 |
|
Stock-based compensation |
|
|
158 |
|
|
|
50 |
|
|
|
108 |
|
Depreciation and amortization |
|
|
121 |
|
|
|
— |
|
|
|
121 |
|
Total |
|
|
2,590 |
|
|
|
469 |
|
|
|
2,121 |
|
1 Total third-party research and development expenses is calculated before salaries, depreciation, amortization and stock-based compensation. Because there is no standard method endorsed by GAAP, the results may not be comparable to similar measurements presented by other public companies.
Total third-party research and development expenses before salaries and benefits, depreciation, amortization and stock-based compensation expenses for the three months ended June 30, 2022, totalled $1,822 compared to $125 for the three months ended June 30, 2021. This increase of $1,697 related mostly to the initiation of clinical development programs GTX 104, GTX 102 and GTX 101, which we acquired through the merger with Grace.
Third-party contract research expenses related to GTX-104 amounted to $323 for the three months ended June 30, 2022, as our PK bridging study progressed. Third party contract research expenses of $567 for GTX-102 are a related to the progression of our CMC Phase 1 work for the manufacturing of clinical trial materials. Third party contract research expenses of $637 for GTX-101 were mostly related to the planning and initiation of the phase 1 single dose study. Other third-party contract research expenses of $154 increased by $78 from $76 for the three months ended June 30, 2021, due to increased IP legal costs to support and maintain our patents that support our three drug candidates GTX-104, GTX-102 and GTX-101. Other expenses for the three months ended June 30, 2021, related to third-party contract research expenses incurred through the completion and termination of the TRILOGY Phase 3 clinical program for CaPre. Professional fees of $243, increased by $227 from $16 for the three months ended June 30, 2021, related to increased specialized consultants supporting our clinical programs for GTX-104, GTX-102 and GTX-101. Third-party research and development expenses were reduced by $190 for the three months ended June 30, 2022, by the increased government credit eligible research activities related to our clinical programs for GTX-104, GTX-102 and GTX-101.
Salaries and benefits increased by $195 to $489 for the three months ended June 30, 2022, from $294 for the three months ended June 30, 2021. The increase is related to additional R&D headcount since the date of the Grace merger, as well as the renewed accruals for our employee incentive bonus program.
General and administrative expenses
General and administrative expenses consist primarily of salaries and related benefits, including share-based compensation, related to our executive, finance, legal, and support functions and other general and administrative expenses include professional fees for auditing, tax, consulting, rent and utilities and insurance.
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Three Months Ended |
|
|
|
|
||||||
|
|
June 30, |
|
|
June 30, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Salaries and benefits |
|
|
532 |
|
|
|
327 |
|
|
|
205 |
|
Professional fees |
|
|
603 |
|
|
|
1,941 |
|
|
|
(1,338 |
) |
Other |
|
|
456 |
|
|
|
305 |
|
|
|
151 |
|
General and administrative expense before stock-based compensation and depreciation 1 |
|
|
1,591 |
|
|
|
2,573 |
|
|
|
(982 |
) |
Stock-based compensation |
|
|
282 |
|
|
|
103 |
|
|
|
179 |
|
Depreciation |
|
|
46 |
|
|
|
— |
|
|
|
46 |
|
Total |
|
|
1,919 |
|
|
|
2,676 |
|
|
|
(757 |
) |
1 General and administrative sub-total expenses is calculated before stock-based compensation and depreciation. Because there is no standard method endorsed by GAAP, the results may not be comparable to similar measurements presented by other public companies.
General and administrative expenses totaled $1,591 before stock-based compensation and depreciation expense for the three months ended June 30, 2022, a decrease of $982 from $2,573 for the three months ended June 30, 2021. This decrease was a result of decreased legal, tax, accounting and other professional fees related to the Grace merger, and the renewal of our at-the-market program that took place during the three months ended June 30, 2021. The decrease in professional fees was partially offset by an increase in salaries and benefits of $205 due to the renewed accrual for our employee incentive bonus program.
Sales and marketing
30
Sales and marketing expenses consist primarily of salaries and benefits, including share-based compensation, related to our commercial functions.
Sales and marketing expenses |
|
|
|
|
|
|
|
|
|
|||
|
|
Three Months Ended |
|
|
|
|
||||||
|
|
June 30, |
|
|
June 30, |
|
|
Increase (Decrease) |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Salaries and benefits |
|
|
182 |
|
|
|
— |
|
|
|
182 |
|
Professional fees |
|
|
5 |
|
|
|
— |
|
|
|
5 |
|
Other |
|
|
10 |
|
|
|
— |
|
|
|
10 |
|
Sub-total |
|
|
197 |
|
|
|
— |
|
|
|
197 |
|
Stock-based compensation |
|
|
24 |
|
|
|
— |
|
|
|
24 |
|
Total |
|
|
221 |
|
|
|
— |
|
|
|
221 |
|
1 Sales and marketing sub-total expenses is calculated before stock-based compensation. Because there is no standard method endorsed by GAAP, the results may not be comparable to similar measurements presented by other public companies.
Sales and marketing expenses before stock-based compensation expense were $197 for the three months ended June 30, 2022 compared to nil for the three months ended June 30, 2021. The increase of $197 was mostly due to an increase in salaries of $182 associated with added personnel.
Aggregate stock-based compensation expense increased by $311 to $464, for the three months ended June 30, 2022, as compared to $1,174 for the three months ended June 30, 2021. This increase was due to the timing of the stock options granted during the year ended March 31, 2022 and year ended March 31, 2021.
Aggregate depreciation expense increased by $167 for the three months ended June 30, 2022, from nil for the three months ended June 30, 2021. This increase is due to the impact of certain equipment being reclassified from held for sale to held for use during the three months ended June 30, 2022 resulting in additional depreciation being recognized.
Liquidity and Capital Resources
Share Capital Structure
Our authorized share capital consists of an unlimited number of Class A, Class B, Class C, Class D and Class E shares, without par value. Issued and outstanding fully paid shares, stock options, and warrants, were as follows for the periods ended (after giving effect to our 8:1 share consolidation, which became effective on August 31, 2021):
|
|
June 30, |
|
|
March 31, |
|
||
|
|
Number |
|
|
Number |
|
||
Class A shares, voting, participating and without par value |
|
|
44,494,193 |
|
|
|
44,288,183 |
|
Stock options granted and outstanding |
|
|
4,227,505 |
|
|
|
2,989,381 |
|
May 2018 Canadian public offering of warrants exercisable at CAD$10.48 until May 9, 2023 |
|
|
824,218 |
|
|
|
824,218 |
|
December 2017 U.S. public offering of warrants exercisable at US$10.08 until December 19, 2022 |
|
|
884,120 |
|
|
|
884,120 |
|
December 2017 U.S. public offering broker warrants exercisable at US$10.10 until December 27, 2022 |
|
|
32,390 |
|
|
|
32,390 |
|
|
|
|
|
|
|
|
||
Total fully diluted shares |
|
|
50,462,426 |
|
|
|
49,018,292 |
|
Cash Flows and Financial Condition for the three months ended June 30, 2022 and 2021
Summary
As at June 30, 2022, cash and cash equivalents totaled $38,377, a net decrease of $2,598 compared to cash and cash equivalents totaling $40,975 at June 30, 2021.
Operating activities
During the three months ended June 30, 2022 and 2021, our operating activities used cash of $5,426 and $3,401, respectively.
Investing activities
During the three months ended June 30, 2022, our investing activities generated cash of $13,258, compared to cash used of $6,927for the three months ended June 30, 2021. The increase in cash generated was a function of an increase in proceeds from maturity of short term investments.
Financing activities
During the three months ended June 30, 2022, our financing activities provided cash totaling $195, compared to cash generated of nil during the three months ended June 30, 2021, due to proceeds from the sale of shares under our ATM program.
31
ATM program
On June 29, 2020, we entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend our ATM program. Under the terms of the Sales Agreement, which has a three-year term, we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. Subject to the terms and conditions of the Sales Agreement, the Agents will use their commercially reasonable efforts to sell the common shares from time to time, based upon our instructions. We have no obligation to sell any of the common shares and may at any time suspend sales under the Sales Agreement. We and the Agents may terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, we have provided the Agents with customary indemnification rights and the Agents will be entitled to compensation at a commission rate equal to 3.0% of the gross proceeds from each sale of the common shares.
On November 10, 2021, we filed a prospectus supplement relating to our ATM program to restore available capacity to $75,000,000, with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC continuing to act as Agents. Under the terms of the Sales Agreement and the prospectus supplement, we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. The common shares will be distributed at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The volume and timing of sales under the ATM program, if any, will be determined at the sole discretion of our board of directors and management. Costs incurred relating to prospectus supplement were $198 and are included in General and administrative expenses.
During the three months ended June 30, 2022, 206,010 common shares were sold for total net proceeds of approximately $195, with commissions, legal expenses and costs related to the share sale amounting to $6. The common shares were sold at the prevailing market prices, which resulted in an average price of approximately $0.97 per share.
Financial Position
The following table details the significant changes to the statements of financial position as at June 30, 2022, compared to the prior fiscal year end at March 31, 2022:
Accounts |
|
Increase |
|
|
Comments |
|
Cash and cash equivalents |
|
|
8,038 |
|
|
See cash flow statement |
Investments |
|
|
(13,306 |
) |
|
Maturity of investments |
Receivables |
|
|
416 |
|
|
Timing of reimbursement of sales taxes |
Prepaid expenses |
|
|
819 |
|
|
Renewal of insurance contract and other prepaid expenses (advances to US vendors) |
Right of use asset |
|
|
504 |
|
|
Adjustment to the net present value of lease contract for Sherbrooke |
Equipment |
|
|
(160 |
) |
|
Depreciation of equipment put back in use |
Trade and other payables |
|
|
(94 |
) |
|
Timing of payments net of accruals |
Lease liability |
|
|
524 |
|
|
Future obligations offset by payment of lease liability |
Derivative warrant liabilities |
|
|
(10 |
) |
|
Change in fair value of derivative warrants |
Deferred tax liability |
|
|
(242 |
) |
|
Related to acquisition of Grace |
See the statement of changes in equity in our financial statements for details of changes to the equity accounts during the three months ended June 30, 2022 and 2021.
Treasury Operations
Our treasury policy is to invest cash that is not required immediately into instruments with an investment strategy based on capital preservation. Cash equivalents and marketable securities are primarily made in guaranteed investment certificates, term deposits and high-interest savings accounts, which are issued and held with Canadian chartered banks, highly-rated promissory notes issued by government bodies and commercial paper. We hold cash denominated in both U.S. and CAD dollars. Funds received in U.S. dollars from equity financings are invested as per our treasury policy in U.S. dollar investments and converted to CAD dollars as appropriate to fulfill operational requirements and funding.
Intangible assets
On August 27, 2021, we completed the acquisition of all outstanding equity interests in Grace Therapeutics Inc, via a merger. Grace, based in New Jersey and organized under the laws of Delaware, was a rare and orphan disease specialty pharmaceutical company.
In connection with the share-for-share noncash transaction, Grace was merged with a new wholly owned subsidiary of Acasti and became a subsidiary of Acasti. As a result, we acquired Grace’s entire therapeutic pipeline consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of various granted and pending patents in various jurisdictions worldwide. Under the terms of the acquisition, each issued and outstanding share of Grace common stock was automatically converted into the right to receive Acasti common shares equal to the equity exchange ratio set forth in the merger agreement.
Intangible assets of $69,810 relate to the value of IPR&D, related to Grace’s therapeutic pipeline, consisting of three unique clinical stage programs/assets supported by intellectual property, the value of which has been attributed as follows:
32
|
|
$ |
|
|
Intangible assets – in-process research and development |
|
|
|
|
GTX-104 |
|
|
27,595 |
|
GTX-102 |
|
|
31,908 |
|
GTX-101 |
|
|
10,307 |
|
Total |
|
|
69,810 |
|
Assets Held for Sale
We determined to actively market for sale Other assets and Production equipment and have met the criteria for classification of assets held for sale:
|
|
June 30, |
|
|
March 31, |
|
||
|
|
|
|
|
Reclassed as explained below |
|
||
|
|
$ |
|
|
$ |
|
||
Other assets (a) |
|
|
195 |
|
|
|
195 |
|
Production equipment (b) |
|
|
157 |
|
|
|
157 |
|
|
|
|
352 |
|
|
|
352 |
|
Other assets
Other assets represent krill oil (“RKO”) held by us that was expected to be used in commercial inventory scale up related to the development and commercialization of the CaPre drug candidate. Given that the development of CaPre will no longer be pursued by us, we expect to sell this reserve. The Other assets is being recorded at the fair value less cost to sell. Management’s estimate of the fair value of the RKO less cost to sell was based primarily on estimated market prices obtained from an appraiser specializing in the krill oil market. These projections are based on Level 3 inputs of the fair value hierarchy and reflect management’s best estimate of market participants’ pricing of the assets as well as the general condition of the asset.
Production equipment
June 30, 2022 |
|
Cost, net of |
|
|
Accumulated |
|
|
Net book |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Production equipment |
|
|
1,179 |
|
|
|
(1,022 |
) |
|
|
157 |
|
|
|
|
1,179 |
|
|
|
(1,022 |
) |
|
|
157 |
|
The announcement of the discontinuation of the CaPre program resulted in an impairment trigger for the related laboratory and production equipment. The impairment loss is based on management’s estimate of the fair value of the equipment less cost to sell, which is based primarily on estimated market prices obtained from brokers specialized in selling used equipment. These projections are based on Level 3 inputs of the fair value hierarchy and reflect our best estimate of market participants’ pricing of the assets as well as the general condition of the assets.
During the three months ended June 30, 2022 we reclassed the following assets from assets held for sale as they no longer met the criteria of such classification.
|
|
Cost, net of |
|
|
Accumulated |
|
|
Net |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Furniture and office equipment |
|
|
17 |
|
|
|
(5 |
) |
|
|
12 |
|
Computer equipment |
|
|
94 |
|
|
|
(6 |
) |
|
|
88 |
|
Laboratory equipment |
|
|
585 |
|
|
|
(435 |
) |
|
|
150 |
|
|
|
|
696 |
|
|
|
(446 |
) |
|
|
250 |
|
Furthermore, depreciation expense of $167 was recognized related to the period from the date that the assets were classified as held for sale until the end of the current period. The reclassification from held for sale to equipment was reflected on the comparative balance sheet. .
Contractual Obligations and Commitments
A summary of the contractual obligations at June 30, 2022, is as follows:
Contractual Obligations |
|
Total |
|
|
Less than |
|
|
1 to 3 years |
|
|
More than |
|
||||
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Trade and other payables |
|
|
3,062 |
|
|
|
3,062 |
|
|
|
— |
|
|
|
— |
|
Operating lease obligations |
|
|
898 |
|
|
|
207 |
|
|
|
394 |
|
|
|
297 |
|
RKO supply agreement |
|
|
2,800 |
|
|
|
2,800 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total |
|
|
6,760 |
|
|
|
6,069 |
|
|
|
394 |
|
|
|
297 |
|
33
Research and development contracts and contract research organizations agreements:
We utilize contract manufacturing organizations, for the development and production of clinical materials and contract research organizations to perform services related to our clinical trials. Pursuant to the agreements with these contract manufacturing organizations and contract research organizations, we have either the right to terminate the agreements without penalties or under certain penalty conditions.
RKO supply agreement
On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antartic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre for a total fixed value of $3.1 million. As at June 30, 2022, the remaining balance of the commitment with Aker amounts to $2.8 million. During the second calendar quarter of 2022, Aker informed the Corporation that Aker believed it had satisfied the terms of the supply agreement as to their ability to deliver the remaining balance of krill oil product, and that the Corporation was therefore required to accept the remaining product commitment and to pay Aker the $2.8 million balance. The Corporation disagrees with Aker’s position and believes that Aker is not entitled to further payment under the supply agreement. Accordingly, no liability has been recorded. The dispute was unresolved as of June 30, 2022, and remains unresolved. There is uncertainty as to whether the Corporation will be required to make further payment to Aker in connection with the dispute. Additionally, in the event the Corporation is required to accept delivery from Aker of the remaining balance of krill oil product under the supply agreement, there is uncertainty as to whether the Corporation can recover value from the product, which may result in the Corporation incurring a loss on the supply agreement in the near term.
Contingencies
We evaluate contingencies on an ongoing basis and establish loss provisions for matters in which losses are probable and the amount of the loss can be reasonably estimated.
Off-Balance Sheet Arrangements
As of the date of this quarterly report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors, except for the RKO supply agreement.
Use of Estimates and Measurement of Uncertainty
The preparation of our financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities, stock-based compensation, assets held for sale, acquisition of Grace valuation of intangibles and supply agreement. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date and determining which research and development expenses qualify for research and development tax credits and in what amounts. We recognize the tax credits once we have reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and, therefore, could be different from the amounts recorded. Estimates and assumptions are also utilized in the assessment of impairment of deferred financing costs, equipment, and intangibles.
Critical Accounting Policies
Valuation of Intangible Assets
In a business combination, the fair value of IPR&D acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as definite-lived intangible assets or discontinued. If discontinued, the intangible assets will be written off. R&D costs incurred after the acquisition are expensed as incurred.
The estimated fair values of identifiable intangible assets were determined using the multi-period excess earnings method, which is a valuation methodology that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. The projected discounted cash flow models used to estimate the fair value of assets of our IPR&D reflect significant assumptions and are level 3 unobservable data regarding the estimates a market participant would make in order to evaluate a drug development asset, including the following:
Based on our valuation assumptions described above, as at the date of acquisition, varying the discount rate would result in the following range in value attributable to each IPR&D intangible asset, related to Grace’s therapeutic pipeline, assuming that all other variables remain constant.
34
Discount assumption |
|
GTX 104 |
|
GTX 102 |
|
GTX 101 |
|
Total |
|
||||
|
|
$ |
|
$ |
|
$ |
|
$ |
|
||||
19.5% |
|
|
26,842 |
|
|
30,564 |
|
|
9,460 |
|
|
66,866 |
|
19.2% (discount rate used in valuation) |
|
|
27,595 |
|
|
31,908 |
|
|
10,307 |
|
|
69,810 |
|
19.0% |
|
|
28,371 |
|
|
33,305 |
|
|
11,009 |
|
|
72,685 |
|
The valuation of our acquired IPR&D has significant measurement uncertainty given the lack of historical data on which to base assumptions. We engaged a third party valuation firm to assist us with the valuation of the IPR&D. Assumptions are difficult to make accurately and were mainly derived from life science studies, industry data, and peer company information that our management believes represent appropriate comparable data.
Goodwill and indefinite-lived assets are not amortized but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of goodwill could occur if the carrying amount of a reporting unit exceeds the fair value of that reporting unit. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
We test goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If we conclude it is more likely than not that fair value of the reporting unit is less than its carrying amount, a quantitative impairment test is performed. We test indefinite lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount.
If we conclude it is more likely than not that the fair value is less than its carrying amount, a quantitative impairment test is performed. There were no triggering events from the date of acquisition of Grace to the end of the quarter. Our annual impairment test will be performed in the third quarter of the fiscal year.
Measurement of Assets Held for Sale and RKO Supply Agreement
Assets that are classified as held for sale are measured at the lower of their carrying amount or fair value less expected selling costs (“estimated selling price”) with a loss recognized to the extent that the carrying amount exceeds the estimated selling price. The classification is applicable at the date upon which the sale of assets is probable, and the assets are available for immediate sale in their present condition. Assets, once classified as held for sale, are not subject to depreciation or amortization and both the assets and any liabilities directly associated with the assets held for sale are classified as current in our consolidated balance sheets. Subsequent changes to the estimated selling price of assets held for sale are recorded as gains or losses to the consolidated statements of income wherein the recognition of subsequent gains is limited to the cumulative loss previously recognized.
In addition, there is judgement and potential for loss regarding the recognition and measurement of our RKO supply agreement with Aker to purchase raw krill oil product for a committed volume of commercial starting material for CaPre for a total fixed value of $3.1 million, which is described in more detail in note 12 of our financial statements found elsewhere in this quarterly report.
Financial Instruments
Credit Risk
Credit risk is the risk of a loss if a customer or counterparty to a financial asset fails to meet its contractual obligations. We have credit risk relating to cash, cash equivalents and short term investments, which we manage by dealing only with highly rated financial institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents our credit exposure at the reporting date.
Currency Risk
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of our business transactions denominated in currencies other than our functional currency. On April 1, 2022, our functional currency was changed from the Canadian dollar to the US dollar. This change is reflected prospectively in the our financial statements.
Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in our operating results.
Since April 1, 2022 a portion of our expenses, mainly related to research contracts is incurred in Canadian dollars and in Euros, for which no financial hedging is in place.
There is a financial risk related to the fluctuation in the value of the Canadian dollar and the Euro in relation to the U.S. dollar. In order to minimize the financial risk related to the fluctuation in the value of the Canadian dollar in relation to the U.S. dollar, funds continue to be invested as cash and cash equivalents and short-term investments in the Canadian dollar.
The following table provides an indication of our significant foreign exchange currency exposures from functional currency at the following dates:
|
|
June 30, 2022 |
June 30, 2021 |
|
||||||||||
|
|
US |
|
EURO |
|
|
CAD |
|
EURO |
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||
Cash and cash equivalents |
|
|
1,451 |
|
|
— |
|
|
|
48,526 |
|
|
— |
|
Investments |
|
|
16 |
|
|
— |
|
|
|
15,381 |
|
|
— |
|
Trade and other payables |
|
|
(690 |
) |
|
(85 |
) |
|
|
(428 |
) |
|
— |
|
|
|
|
777 |
|
|
(85 |
) |
|
|
63,479 |
|
|
— |
|
35
The following exchange rates are those applicable to the following periods and dates:
|
|
June 30, 2022 |
|
|
June 30, 2021 |
|
||||||||||
|
|
Average |
|
|
Reporting |
|
|
Average |
|
|
Reporting |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
US$ per CAD (2021 - CAD per US $) |
|
|
0.7835 |
|
|
|
0.7768 |
|
|
|
1.2282 |
|
|
|
1.2398 |
|
USD$ per Euro |
|
|
1.0647 |
|
|
|
1.0484 |
|
|
|
1.2052 |
|
|
|
1.1858 |
|
Based on our foreign currency exposures noted above, varying the above foreign exchange rates to reflect a 5% strengthening of the U.S. dollar and Euro would have an increase (decrease) in net loss as follows, assuming that all other variables remain constant:
|
|
June 30, 2021 |
|
|
|
|
$ |
|
|
|
|
|
|
|
Increase (decrease) in net loss |
|
|
3,935 |
|
Based on our foreign currency exposures noted above, varying the above foreign exchange rates to reflect a 5% strengthening of the Canadian dollar and Euro would have an increase (decrease) in net loss as follows, assuming that all other variables remain constant:
|
|
June 30, 2022 |
|
|
|
|
$ |
|
|
|
|
|
|
|
Increase (decrease) in net loss |
|
|
29 |
|
An assumed 5% weakening of the foreign currencies would have an equal but opposite effect on the basis that all other variables remained constant.
Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates. Our exposure to interest rate risk as at June 30, 2022 and 2021 was as follows:
Cash and cash equivalents |
|
Short-term fixed interest rate |
Investments |
|
Short-term fixed interest rate |
Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of our short-term investments is limited because these investments have short-term maturities and are held to maturity.
Liquidity Risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they fall due. We manage liquidity risk through the management of our capital structure and financial leverage. We also manage liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves our operating budgets and reviews material transactions outside the normal course of business.
Our contractual obligations related to financial instruments and other obligations and liquidity resources are presented in the liquidity and capital resources of this MD&A and note 1, Nature of operations in the Financial Statements.
Future Accounting Changes
We have considered recent accounting pronouncements and concluded that they are either not applicable to our business or that the effect is not expected to be material to our consolidated financial statements as a result of future adoption.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Information relating to quantitative and qualitative disclosures about market risks is detailed in “Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
Item 4. Controls and Procedures
Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report, our management, with the participation of our CEO and CFO, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15 (e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of June 30, 2022, our existing disclosure controls and procedures were effective. It should be noted that while our CEO and CFO believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, but not absolute, assurance that the objectives of the control system are met.
36
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the quarter ended June 30, 2022 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
In the ordinary course of business, we are at times subject to various legal proceedings and disputes, including the proceedings specifically discussed below. We assess our liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that we will incur a loss and the amount of the loss can be reasonably estimated, we record a liability in our consolidated financial statements. These legal reserves may be increased or decreased to reflect any relevant developments on a quarterly basis. Where a loss is not probable or the amount of loss is not estimable, we do not accrue legal reserves. While the outcome of legal proceedings is inherently uncertain, based on information currently available and available insurance coverage, our management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on our financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to our financial position, results of operations, or cash flows.
Litigation Related to the Merger
In connection with the Grace merger, four stockholder lawsuits have been filed:
The Complaints generally allege that our public disclosures pertaining to the Grace merger omitted material facts in purported violation of Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder, and further that members of our Board of Directors are liable for those purported omissions under Section 20(a) of the Exchange Act. The relief sought in the Complaints includes, among other things to enjoin the consummation of the merger pending disclosure of sufficient information, to award damages purportedly caused by the alleged omissions, and to award plaintiffs’ attorneys’ fees and other costs.
The Dawson and Weir Complaints have been voluntarily dismissed without prejudice. The Bisel and Castaldo Complaints have been consolidated. The plaintiffs amended their Complaint in the consolidated action on October 1, 2021, to assert their claims on a class wide basis. The court appointed Plaintiff Castaldo as Lead Plaintiff for the putative class in the consolidated action. Castaldo filed an amended Complaint by February 4, 2022. Acasti filed a motion to dismiss on February 25, 2022.
It is possible that additional lawsuits asserting similar claims could be filed. We strongly believe the allegations in the Complaints are frivolous and without merit, and are vigorously defending against them.
Item 1A. Risk Factors
There have been no material changes from the risk factors disclosed in our most recently filed annual report on Form 10-K.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
37
Item 6. Exhibits
Exhibit No. |
|
Description |
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
38
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: August 11, 2022
|
ACASTI PHARMA INC. |
||
|
|
|
|
|
By: |
/s/ Janelle D’Alvise |
|
|
|
Name: Janelle D’Alvise |
|
|
|
Title: President and Chief Executive Officer and Director (Principal Executive Officer) |
|
|
|
|
|
|
By: |
/s/ Brian Ford |
|
|
|
Name: Brian Ford |
|
|
|
Title: Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
39