EXHIBIT 99.2
Published on November 13, 2025
Exhibit 99.2

Corporate Presentation Fall 2025

Summary 2 Forward Looking Statements Statements in this presentation that are
not statements of historical or current fact constitute "forward-looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, Section 27A of the Securities Act of 1933, as amended, and
Section 21E of the Securities Exchange Act of 1934, as amended, and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Such forward-looking statements involve known and
unknown risks, uncertainties, and other factors that could cause the actual results of Grace Therapeutics, Inc. (the “Company”) to be materially different from historical results or from any future results expressed or implied by such
forward-looking statements. In addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements containing the terms "believes," "belief," "expects," "intends," "anticipates,"
"estimates," "potential," "should," "may," "will," "plans," "continue," "targeted" or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which
speak only as of the date of this presentation. The forward-looking statements in this presentation, including, but not limited to, statements regarding the Company’s expected cash runway, the potential exercise of outstanding warrants, the
future prospects of the Company’s GTx-104 drug candidate, the outcome of the Company's NDA submission for GTx-104 with the FDA, GTx-104's potential to bring enhanced treatment options to patients suffering from aneurysmal subarachnoid
hemorrhage (“aSAH”), GTx-104’s potential to be administered to improve the management of hypotension in patients with aSAH, the ability of GTx-104 to achieve a pharmacokinetic and safety profile similar to the oral form of nimodipine,
GTx-104’s potential to provide improved bioavailability and the potential for reduced use of rescue therapies, GTx-104’s potential to achieve pharmacoeconomic benefit over the oral form of nimodipine, GTx-104’s commercial prospects, the
Company’s pre-commercial launch strategy for GTx104, the future prospects of the Company’s GTx-102 drug candidate, GTx-102’s potential to provide clinical benefits to decrease symptoms associated with Ataxia Telangiectasia, the timing and
outcomes of a Phase 3 efficacy and safety trial for GTx-102, the timing of an NDA filing for GTx-102, the future prospects of the Company’s GTx-101 drug candidate, GTx-101’s potential to be administered to Postherpetic Neuralgia (“PHN”)
patients to treat severe nerve pain associated with the disease, the timing and outcomes of a Phase 3 efficacy and safety trial for GTx-101, the size of the addressable market for GTx-104 and GTx 102, and any future patent and other
intellectual property filings made by the Company for new developments are based upon Grace Therapeutics, Inc.’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the
timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions of the
STRIVE-ON Phase 3 safety trial for GTx-104; (ii) regulatory requirements or developments and the outcome of the NDA submission for GTx-104; (iii) changes to clinical trial designs and regulatory pathways; (iv) the Company’s ability to protect
its intellectual property rights for its product candidates; and (v) legislative, regulatory, political and economic developments. The foregoing list of important factors that could cause actual events to differ from expectations should not
be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in the "Special Note Regarding Forward-Looking Statements," "Risk Factors" and
"Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of the Company's Annual Report on Form 10-K for the fiscal year ended March 31, 2025, Quarterly Reports on Forms 10-Q for the quarterly periods
ended June 30, 2025 and September 30, 2025 and other documents that have been and will be filed by Grace Therapeutics, Inc. from time to time with the Securities and Exchange Commission and Canadian securities regulators. All forward-looking
statements contained in this presentation speak only as of the date on which they were made. Grace Therapeutics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on
which they were made, except as required by applicable securities laws.
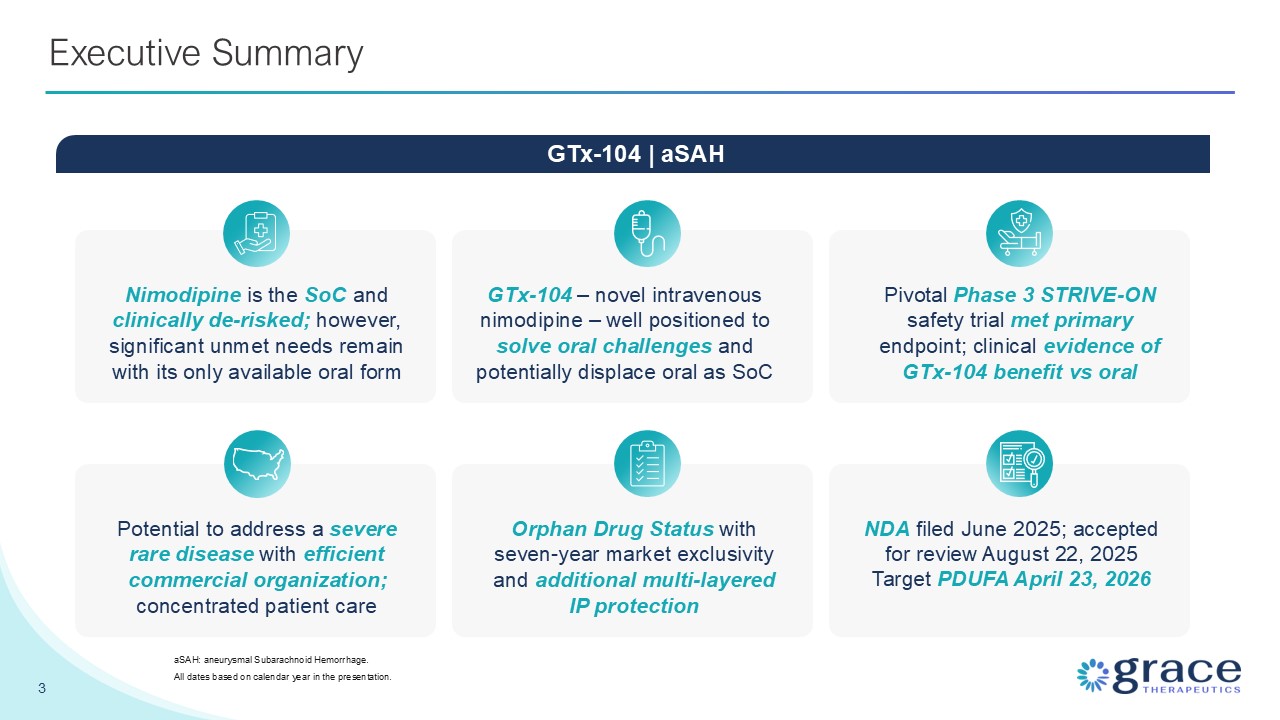
Summary GTx-104 – novel intravenous nimodipine – well positioned to solve oral
challenges and potentially displace oral as SoC Nimodipine is the SoC and clinically de-risked; however, significant unmet needs remain with its only available oral form Pivotal Phase 3 STRIVE-ON safety trial met primary endpoint; clinical
evidence of GTx-104 benefit vs oral 3 Executive Summary aSAH: aneurysmal Subarachnoid Hemorrhage. All dates based on calendar year in the presentation. GTx-104 | aSAH Potential to address a severe rare disease with efficient commercial
organization; concentrated patient care Orphan Drug Status with seven-year market exclusivity and additional multi-layered IP protection NDA filed June 2025; accepted for review August 22, 2025 Target PDUFA April 23, 2026
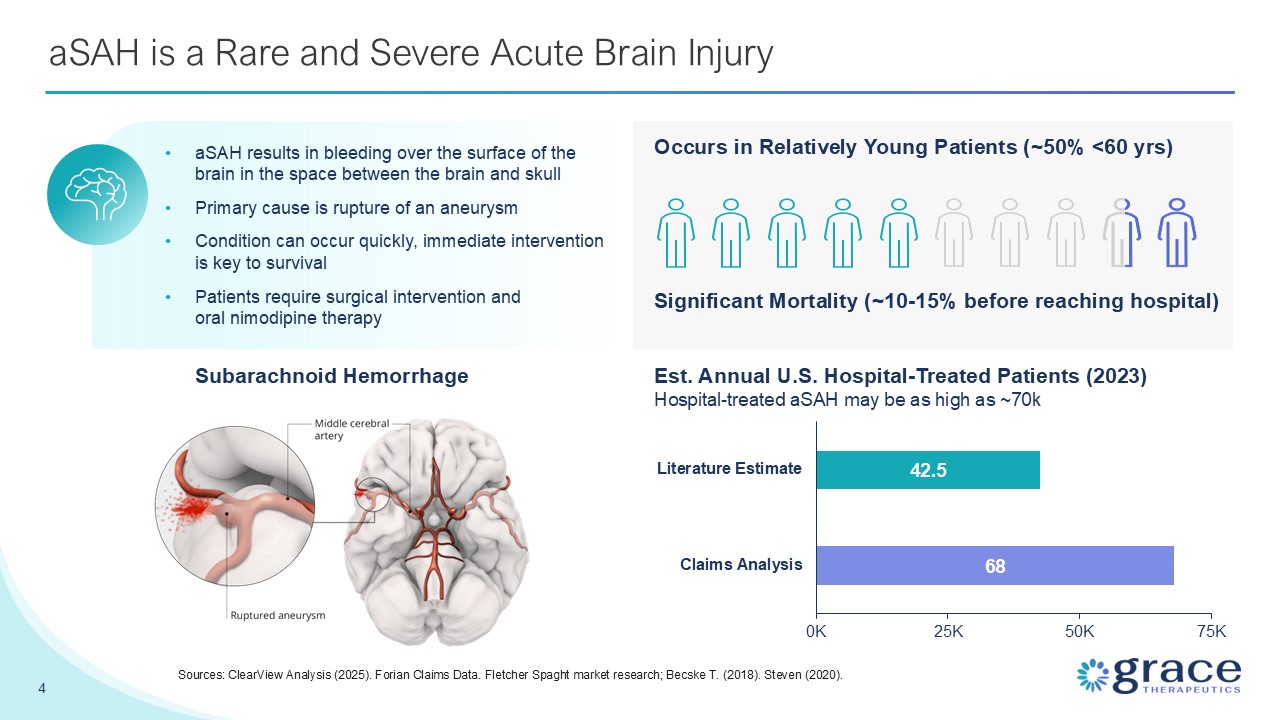
aSAH is a Rare and Severe Acute Brain Injury Subarachnoid Hemorrhage aSAH
results in bleeding over the surface of the brain in the space between the brain and skull Primary cause is rupture of an aneurysm Condition can occur quickly, immediate intervention is key to survival Patients require surgical
intervention and oral nimodipine therapy 4 Sources: ClearView Analysis (2025). Forian Claims Data. Fletcher Spaght market research; Becske T. (2018). Steven (2020). Occurs in Relatively Young Patients (~50% <60 yrs) Significant
Mortality (~10-15% before reaching hospital) Est. Annual U.S. Hospital-Treated Patients (2023) Hospital-treated aSAH may be as high as ~70k

Oral Nimodipine – The aSAH Standard of Care for >3 Decades 5 Sources: Hoh
(2023). Hernandez-Duran (2019). Sandow (2016). DCI: Delayed Cerebral Infarction The Joint Commision is a hospital accredation agency 2023 AHA/ASA GuidelinesFor the management of patients with aSAH Nimodipine is the only approved therapy
to improve neurological outcomes Limited use of off-label therapies due to The Joint Commission monitoring adherence to care guidelines
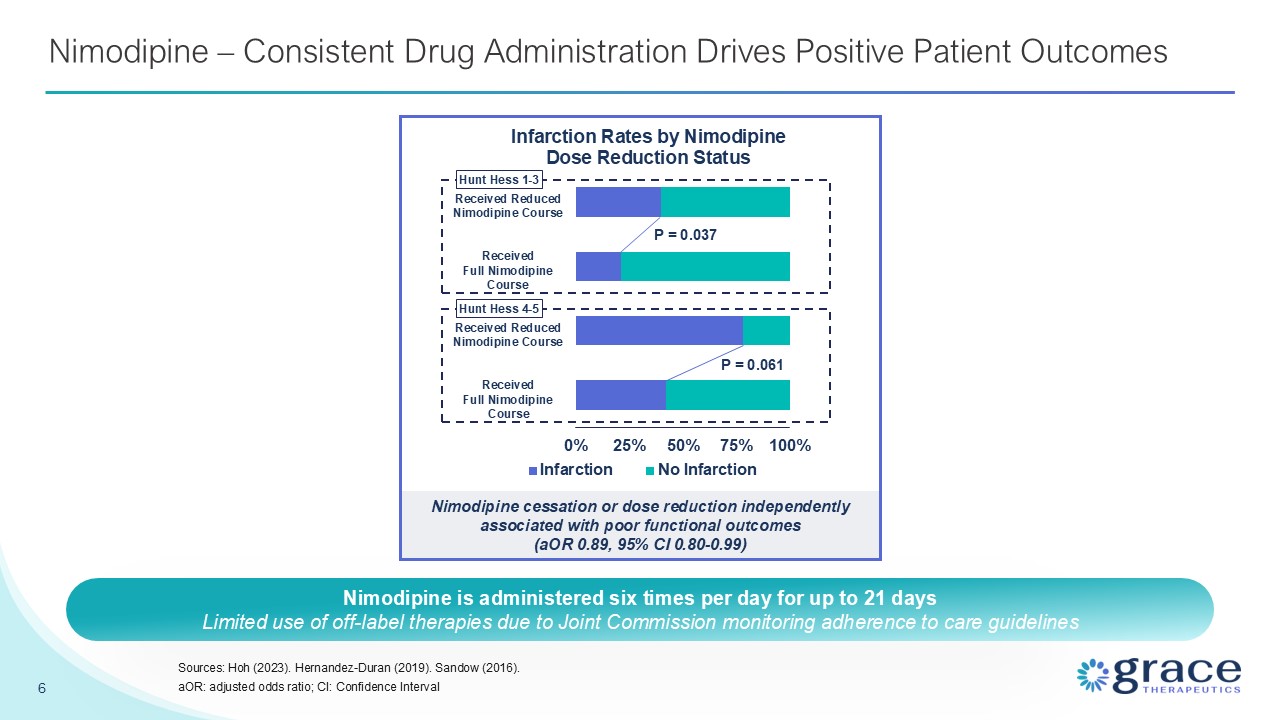
Nimodipine – Consistent Drug Administration Drives Positive Patient
Outcomes 6 Sources: Hoh (2023). Hernandez-Duran (2019). Sandow (2016). aOR: adjusted odds ratio; CI: Confidence Interval Nimodipine cessation or dose reduction independently associated with poor functional outcomes (aOR 0.89, 95% CI
0.80-0.99) P = 0.037 P = 0.061 Received Reduced Nimodipine Course Received Full Nimodipine Course Received Reduced Nimodipine Course Received Full Nimodipine Course Hunt Hess 1-3 Hunt Hess 4-5 Nimodipine is administered six times per
day for up to 21 days Limited use of off-label therapies due to Joint Commission monitoring adherence to care guidelines
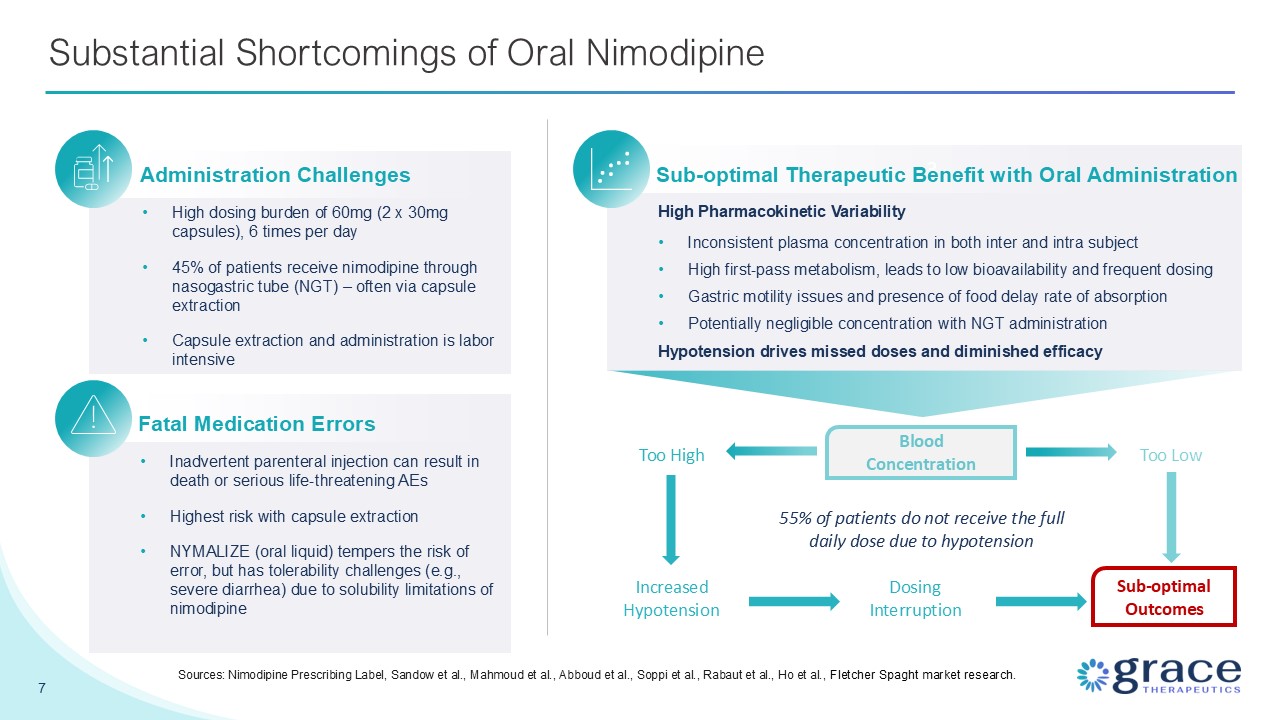
7 Substantial Shortcomings of Oral Nimodipine Sources: Nimodipine Prescribing
Label, Sandow et al., Mahmoud et al., Abboud et al., Soppi et al., Rabaut et al., Ho et al., Fletcher Spaght market research. Administration Challenges High dosing burden of 60mg (2 x 30mg capsules), 6 times per day 45% of patients receive
nimodipine through nasogastric tube (NGT) – often via capsule extraction Capsule extraction and administration is labor intensive Dosing Interruption Increased Hypotension Too High Fatal Medication Errors Inadvertent parenteral
injection can result in death or serious life-threatening AEs Highest risk with capsule extraction NYMALIZE (oral liquid) tempers the risk of error, but has tolerability challenges (e.g., severe diarrhea) due to solubility limitations of
nimodipine 3 Sub-optimal Therapeutic Benefit with Oral Administration High Pharmacokinetic Variability Inconsistent plasma concentration in both inter and intra subject High first-pass metabolism, leads to low bioavailability and
frequent dosing Gastric motility issues and presence of food delay rate of absorption Potentially negligible concentration with NGT administration Hypotension drives missed doses and diminished efficacy Blood Concentration 55% of
patients do not receive the full daily dose due to hypotension Sub-optimal Outcomes Too Low
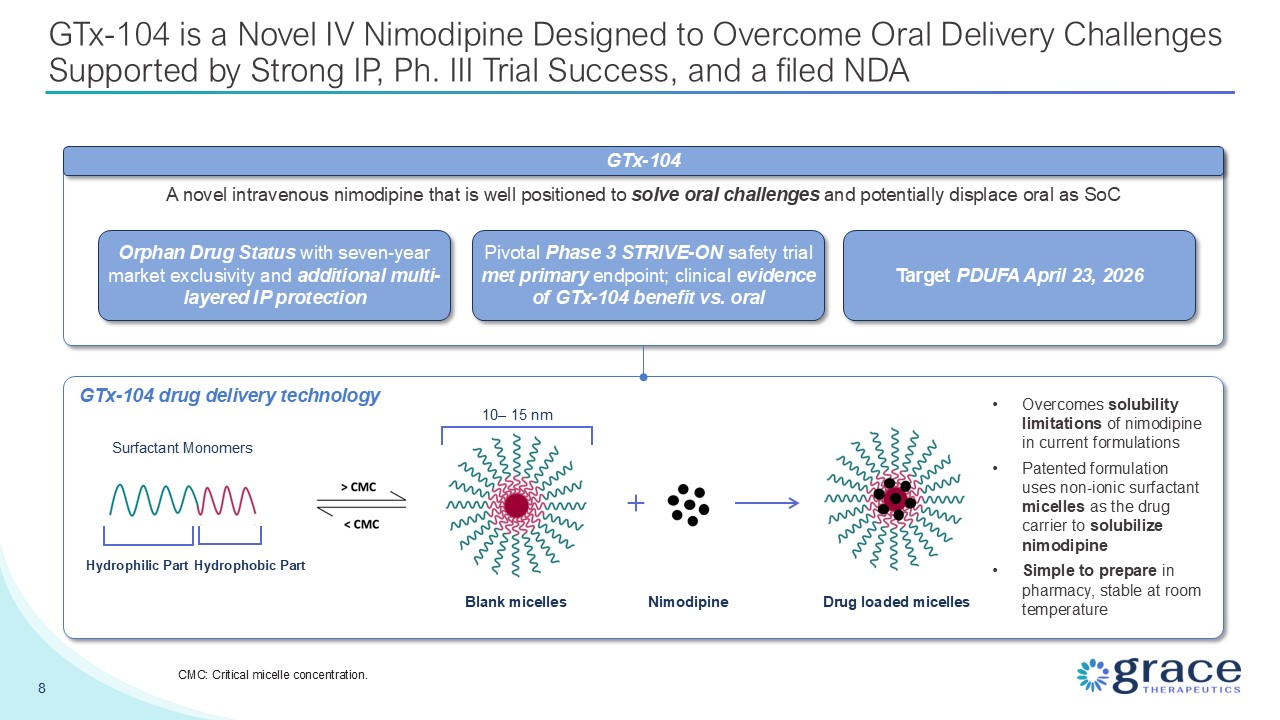
GTx-104 is a Novel IV Nimodipine Designed to Overcome Oral Delivery Challenges
Supported by Strong IP, Ph. III Trial Success, and a filed NDA 8 CMC: Critical micelle concentration. Orphan Drug Status with seven-year market exclusivity and additional multi-layered IP protection Drug loaded micelles Nimodipine 10–
15 nm Blank micelles Surfactant Monomers Hydrophilic Part Hydrophobic Part A novel intravenous nimodipine that is well positioned to solve oral challenges and potentially displace oral as SoC Pivotal Phase 3 STRIVE-ON safety trial met
primary endpoint; clinical evidence of GTx-104 benefit vs. oral Target PDUFA April 23, 2026 GTx-104 Overcomes solubility limitations of nimodipine in current formulations Patented formulation uses non-ionic surfactant micelles as the
drug carrier to solubilize nimodipine Simple to prepare in pharmacy, stable at room temperature GTx-104 drug delivery technology

GTx-104 Value Proposition 9 Risk of Fatal Parenteral Use Requires Feeding
Tube Excipient Intolerance Hemodynamic Control Dose Compliance Markets Nimodipine Capsules Yes Yes No Poor Poor U.S. / WW NYMALIZE (Oral Liquid) Yes (Reduced) Yes Yes Poor Poor U.S. / Select WW NIMOTOP
(Injectable) No No Yes * Unknown Rescue Only EU / China GTx-104 No No No Optimal Optimal Global Rights NDA Submission 1H:25 Sources: Nimodipine capsule packaging insert. Fletcher Spaght market research. Soppi V. (2007). *
High alcohol content (~24% volume/volume) also requires central catheter for administration WW: Worldwide DDI: drug-drug internation Predictable drug concentration & dose compliance Reduced drug intake, reduced DDIs & no food
effects More effective hypotension management Clinical Value Hospital Value Reduced hospital resources The Joint Commission compliance to aSAH care guidelines Reduced medication errors & nursing burden Patient Value Lower disease
burden & faster recovery Safer & more convenient treatment Improved functional outcomes
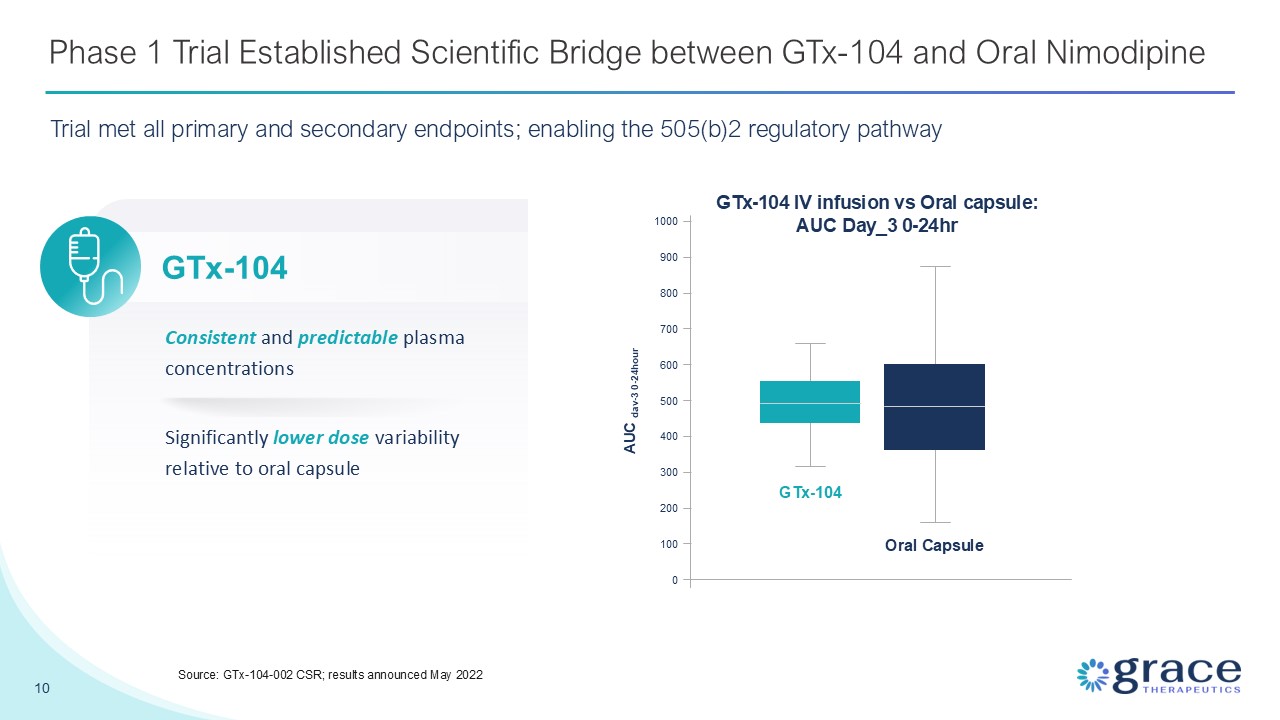
Phase 1 Trial Established Scientific Bridge between GTx-104 and Oral
Nimodipine 10 Source: GTx-104-002 CSR; results announced May 2022 Significantly lower dose variability relative to oral capsule Consistent and predictable plasma concentrations GTx-104 IV infusion vs Oral capsule: AUC Day_3
0-24hr GTx-104 Oral Capsule 0 100 200 300 400 500 600 700 800 900 1000 AUC dav-3 0-24hour GTx-104 Trial met all primary and secondary endpoints; enabling the 505(b)2 regulatory pathway

STRIVE-ON Phase 3 Trial
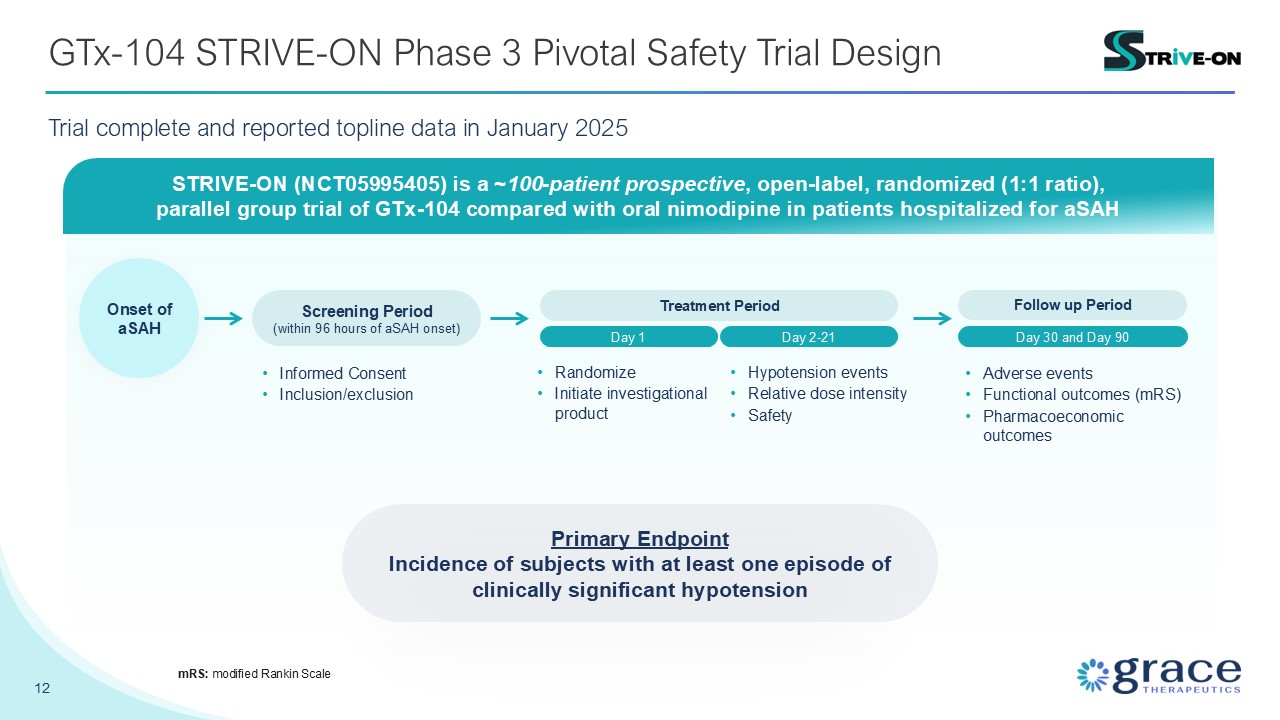
GTx-104 STRIVE-ON Phase 3 Pivotal Safety Trial Design 12 mRS: modified Rankin
Scale STRIVE-ON (NCT05995405) is a ~100-patient prospective, open-label, randomized (1:1 ratio), parallel group trial of GTx-104 compared with oral nimodipine in patients hospitalized for aSAH Screening Period (within 96 hours of aSAH
onset) Day 1 Treatment Period Day 2-21 Onset of aSAH Follow up Period Day 30 and Day 90 Primary Endpoint Incidence of subjects with at least one episode of clinically significant hypotension Informed
Consent Inclusion/exclusion Randomize Initiate investigational product Hypotension events Relative dose intensity Safety Adverse events Functional outcomes (mRS) Pharmacoeconomic outcomes Trial complete and reported topline data
in January 2025

13 STRIVE-ON Trial Data Demonstrates Key Clinical, Pharmacoeconomic, and Dosing
/ Administration Benefits over Current SoC, Oral Nimodipine CLINICAL IMPROVED 90-DAY OUTCOMES (MRS*) +29% relative increase in patients with good recovery at 90 days vs. oral nimodipine FEWER HYPOTENSION EVENTS -19% reduction from oral
nimodipine BETTER RELATIVE DOSE INTENSITY 54% vs. 8% with oral nimodipine receive >95% prescribed dose PHARMACOECONOMIC FEWER ICU DAYS -1.5 days reduction from oral nimodipine LESS TIME ON VENTILATION -5 days reduction from
oral nimodipine REDUCED ICU READMISSION RATES -48% reduction from oral nimodipine DOSING & ADMIN. IMPROVED PATIENT REST No need to disrupt patient sleep every 4 hours EASIER ADMINISTRATION No feeding tube or swallowing of
large pills required LESS LABOR-INTENSIVE TREATMENT PREP No nimodipine capsule extraction and administration (laborious for staff) * mRS – modified Rankin Score
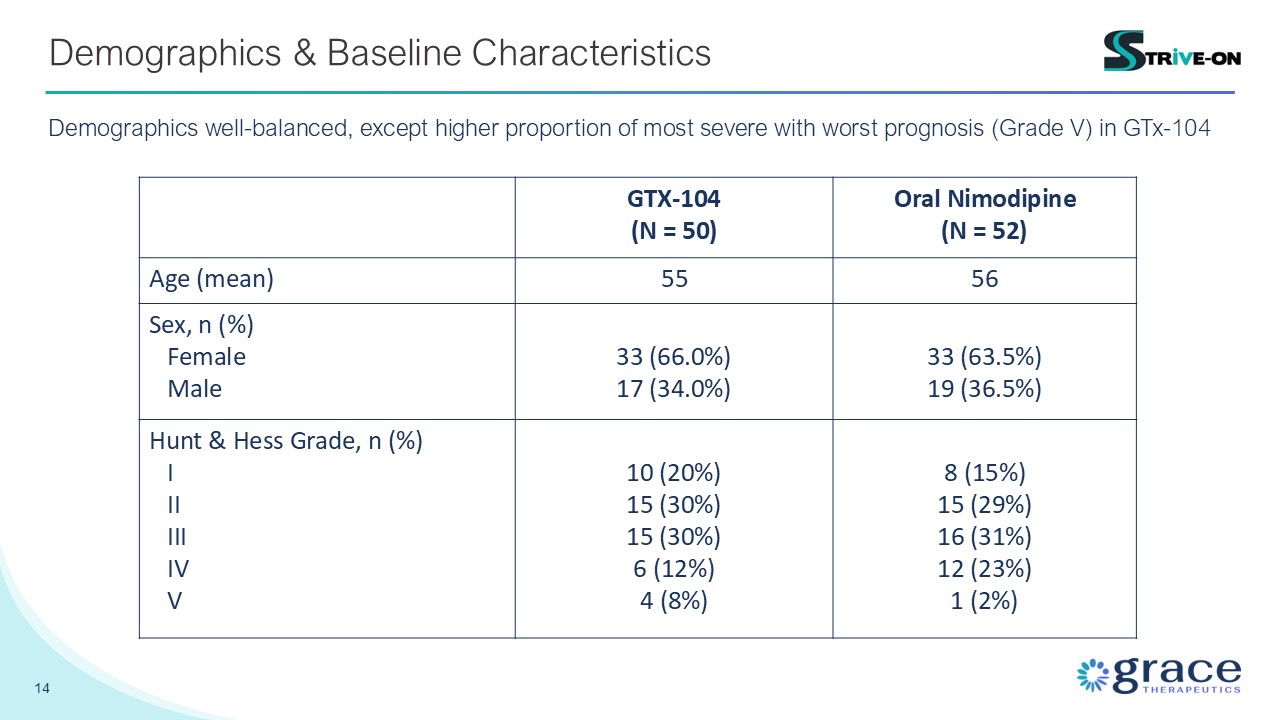
14 Demographics & Baseline Characteristics GTX-104 (N = 50) Oral
Nimodipine (N = 52) Age (mean) 55 56 Sex, n (%) Female Male 33 (66.0%) 17 (34.0%) 33 (63.5%) 19 (36.5%) Hunt & Hess Grade, n (%) I II III IV V 10 (20%) 15 (30%) 15 (30%) 6 (12%) 4 (8%) 8 (15%) 15 (29%) 16
(31%) 12 (23%) 1 (2%) Demographics well-balanced, except higher proportion of most severe with worst prognosis (Grade V) in GTx-104
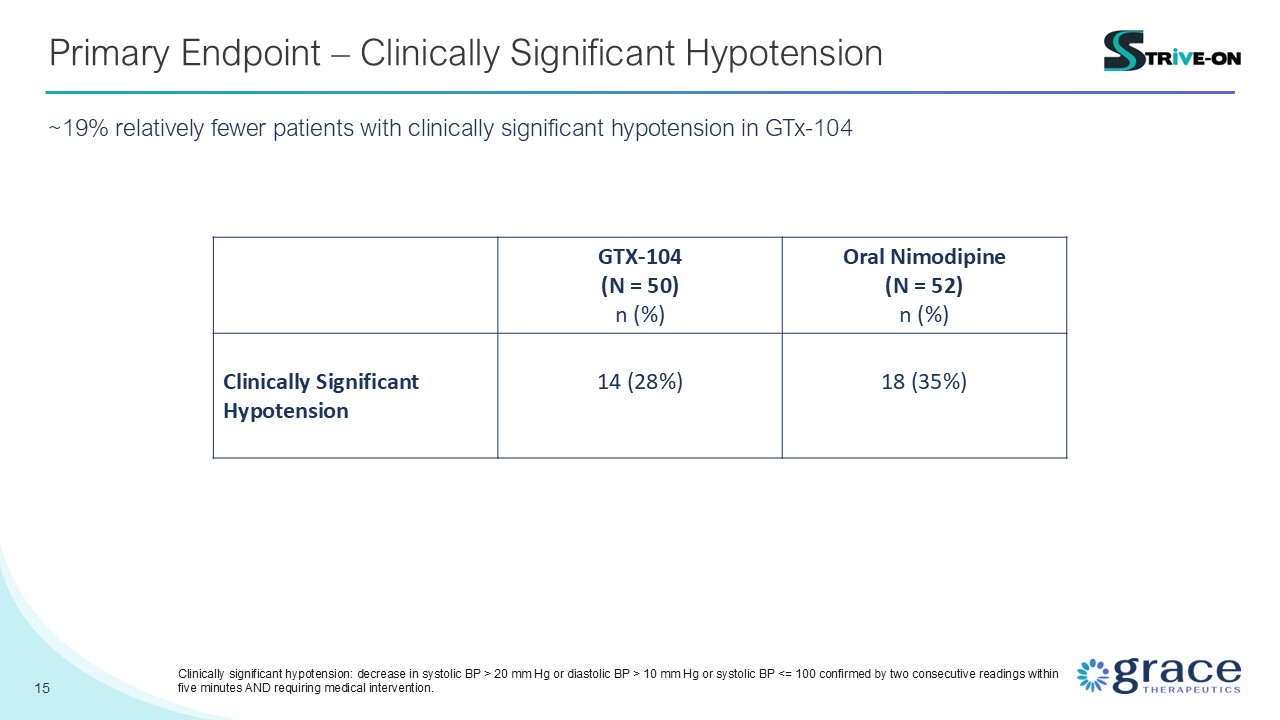
15 Primary Endpoint – Clinically Significant Hypotension ~19% relatively
fewer patients with clinically significant hypotension in GTx-104 GTX-104 (N = 50) n (%) Oral Nimodipine (N = 52) n (%) Clinically Significant Hypotension 14 (28%) 18 (35%) Clinically significant hypotension: decrease in systolic BP
> 20 mm Hg or diastolic BP > 10 mm Hg or systolic BP <= 100 confirmed by two consecutive readings within five minutes AND requiring medical intervention.
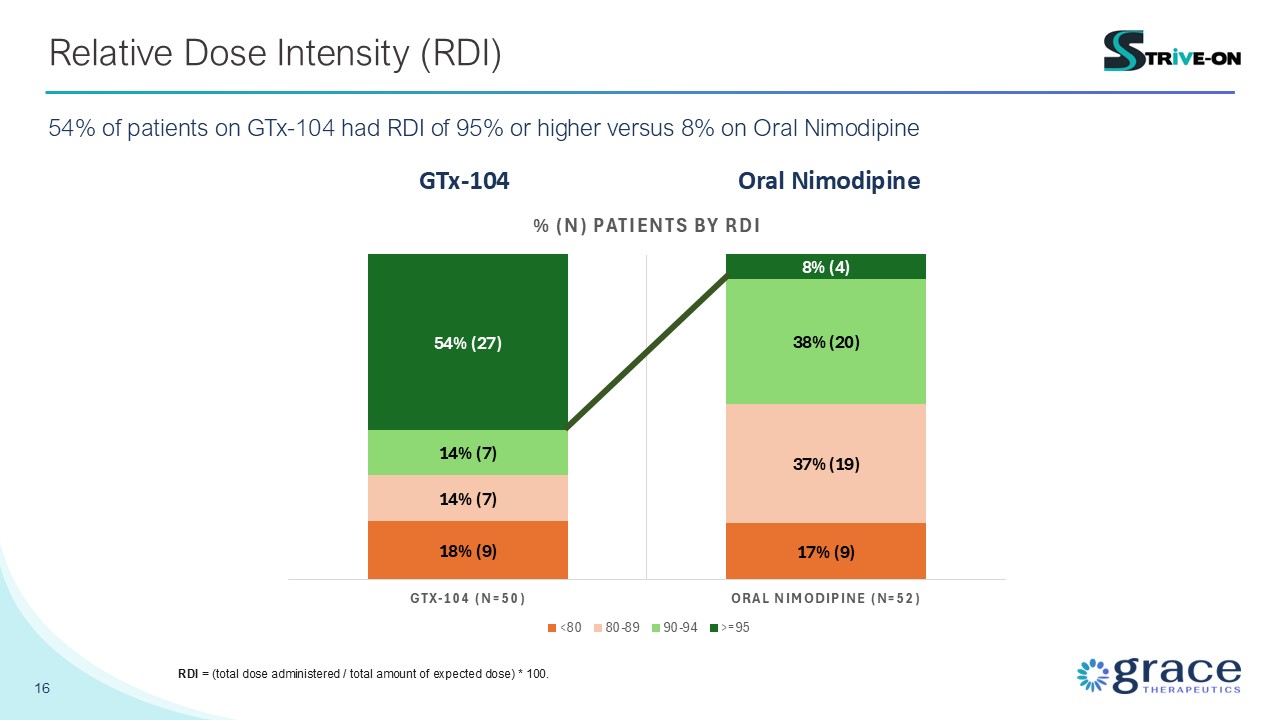
16 Relative Dose Intensity (RDI) 54% of patients on GTx-104 had RDI of 95% or
higher versus 8% on Oral Nimodipine GTx-104 Oral Nimodipine RDI = (total dose administered / total amount of expected dose) * 100.
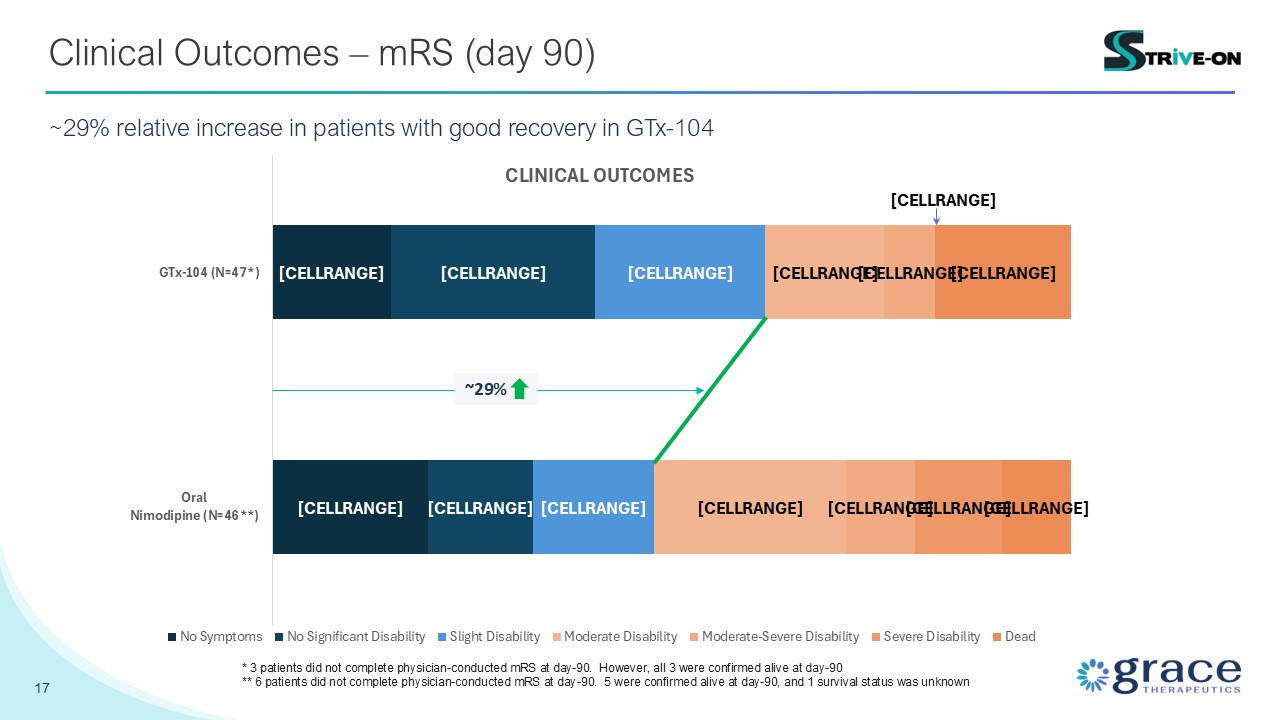
17 Clinical Outcomes – mRS (day 90) ~29% relative increase in patients with
good recovery in GTx-104 ~29% * 3 patients did not complete physician-conducted mRS at day-90. However, all 3 were confirmed alive at day-90 ** 6 patients did not complete physician-conducted mRS at day-90. 5 were confirmed alive at
day-90, and 1 survival status was unknown
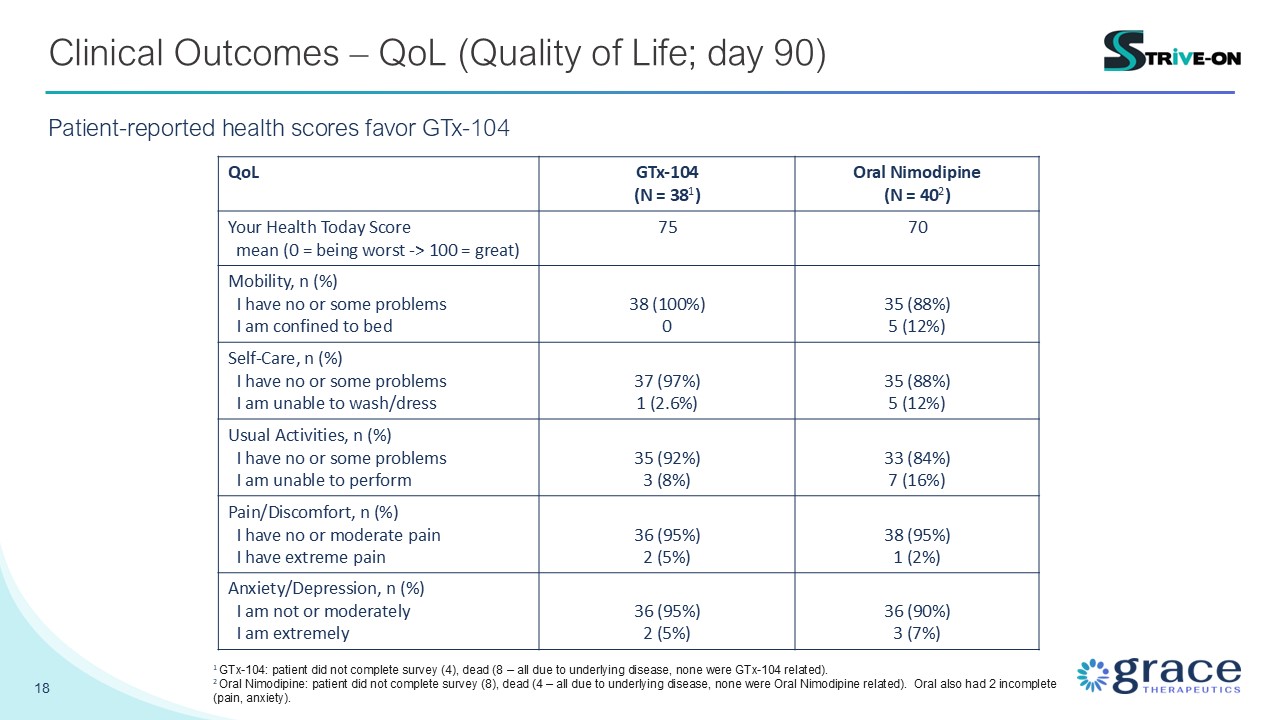
18 Clinical Outcomes – QoL (Quality of Life; day 90) Patient-reported health
scores favor GTx-104 QoL GTx-104 (N = 381) Oral Nimodipine (N = 402) Your Health Today Score mean (0 = being worst -> 100 = great) 75 70 Mobility, n (%) I have no or some problems I am confined to bed 38 (100%) 0 35
(88%) 5 (12%) Self-Care, n (%) I have no or some problems I am unable to wash/dress 37 (97%) 1 (2.6%) 35 (88%) 5 (12%) Usual Activities, n (%) I have no or some problems I am unable to perform 35 (92%) 3 (8%) 33 (84%) 7
(16%) Pain/Discomfort, n (%) I have no or moderate pain I have extreme pain 36 (95%) 2 (5%) 38 (95%) 1 (2%) Anxiety/Depression, n (%) I am not or moderately I am extremely 36 (95%) 2 (5%) 36 (90%) 3 (7%) 1 GTx-104:
patient did not complete survey (4), dead (8 – all due to underlying disease, none were GTx-104 related). 2 Oral Nimodipine: patient did not complete survey (8), dead (4 – all due to underlying disease, none were Oral Nimodipine related).
Oral also had 2 incomplete (pain, anxiety).

19 Safety Overall safety was comparable between the two groups Summary of
Adverse Events (AEs) (entire study duration of 90 days) GTx-104 (N = 50) Oral Nimodipine (N = 52) All AEs, n (%) # of events 44 (88%) 157 43 (83%) 193 All AEs, events per n 3.6 4.5 All SAEs1, n (%) # of events 18 (36%) 34 25
(48%) 48 All SAEs, events per n 1.9 1.9 Treatment-Related SAEs, n (%) # of events2 0 2 (4%) 2 Mortality3, n (%) 8 (16%) 4 (8%) Cause of death4 (n) All deaths were due to severity of underlying disease No deaths due to
GTx-104 aSAH (5), ICH (1), rebleed (1), cardiac arrest (1) No deaths due to Oral Nimodipine aSAH (2), rebleed (1), cardiac arrest (1) 1 A few include sepsis, deep vein thrombosis, ICH, hydrocephalus, cerebral infarction, urinary tract
infection, C. difficile, systemic inflammatory response, acute kidney injury, as well as death 2 Oral Nimodipine: bradycardia, vasospasm 3 Mortality rate is equivalent or lower than previous well-controlled clinical trials (Oral NIMOTOP
NDA) 4 Based on investigator assessment SAEs: Serious Adverse Events; ICH: Intracerebral Hemorrhage; DCI: Delayed Cerebral Hemorrhage
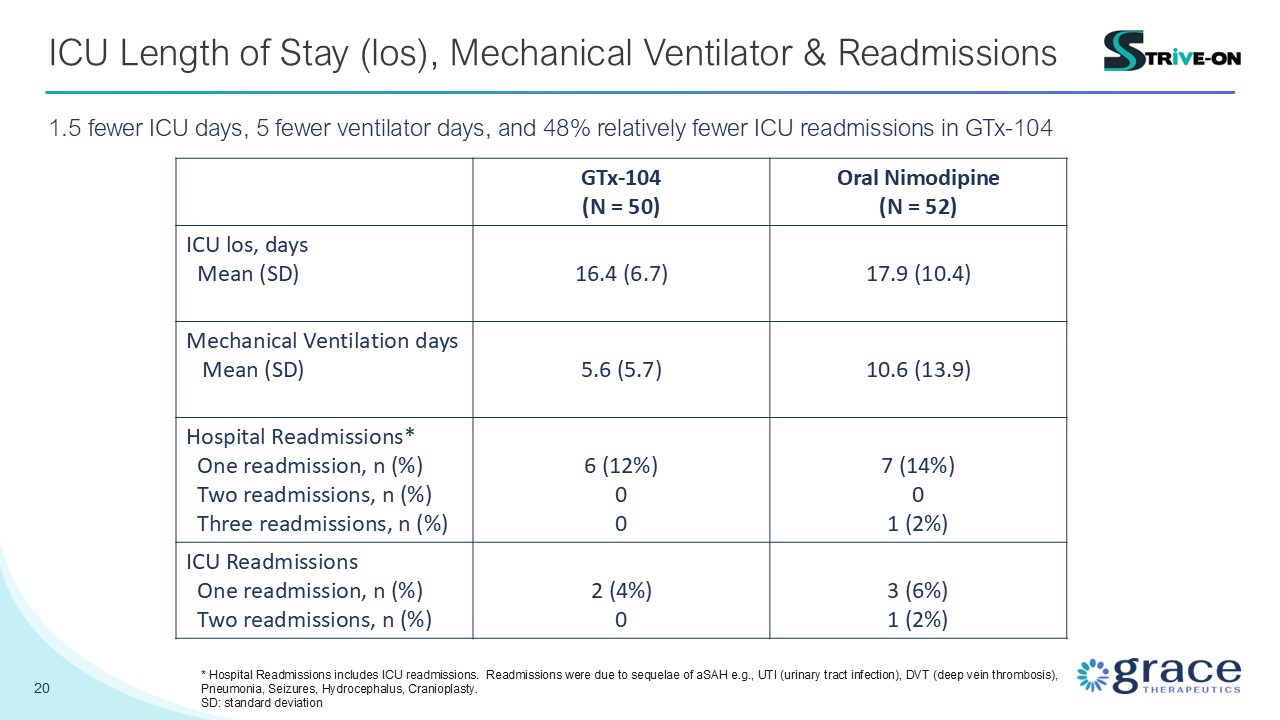
20 ICU Length of Stay (los), Mechanical Ventilator & Readmissions 1.5
fewer ICU days, 5 fewer ventilator days, and 48% relatively fewer ICU readmissions in GTx-104 GTx-104 (N = 50) Oral Nimodipine (N = 52) ICU los, days Mean (SD) 16.4 (6.7) 17.9 (10.4) Mechanical Ventilation days Mean (SD) 5.6
(5.7) 10.6 (13.9) Hospital Readmissions* One readmission, n (%) Two readmissions, n (%) Three readmissions, n (%) 6 (12%) 0 0 7 (14%) 0 1 (2%) ICU Readmissions One readmission, n (%) Two readmissions, n (%) 2 (4%) 0 3
(6%) 1 (2%) * Hospital Readmissions includes ICU readmissions. Readmissions were due to sequelae of aSAH e.g., UTI (urinary tract infection), DVT (deep vein thrombosis), Pneumonia, Seizures, Hydrocephalus, Cranioplasty. SD: standard
deviation

21 Pharmacoeconomics Major patient resource utilization drivers in aSAH favor
GTx-104 GTx-104 (N = 50) n* Oral Nimodipine (N = 52) n* Day 1 Day 14 % change Day 1 Day 14 % change Mechanical Ventilation 14 1 -93% 12 7 -42% External Ventricular Drain 32 10 -69% 35 17 -51% Deep
Sedation 5 1 -80% 8 5 -38% Comatose 4 0 -100% 5 2 -60% * Excludes patients that died before Day 14 for this analysis.

Commercial Preparation
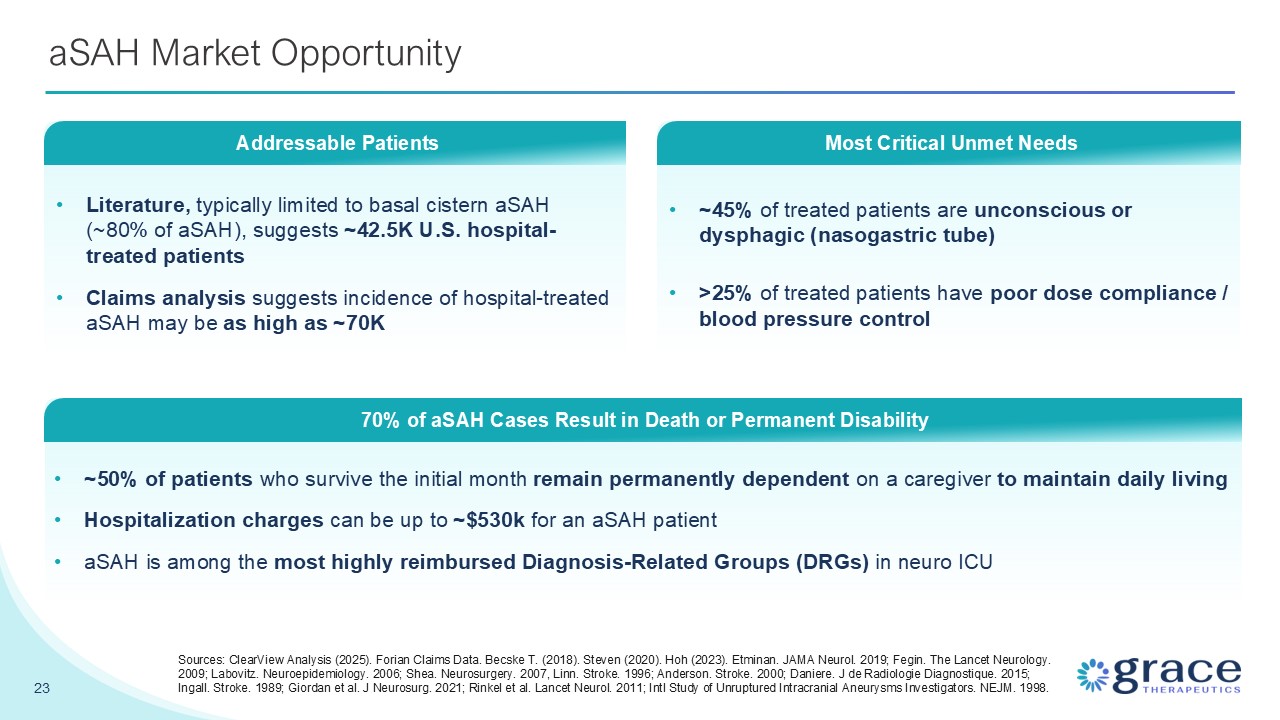
~45% of treated patients are unconscious or dysphagic (nasogastric
tube) >25% of treated patients have poor dose compliance / blood pressure control aSAH Market Opportunity 23 Literature, typically limited to basal cistern aSAH (~80% of aSAH), suggests ~42.5K U.S. hospital-treated patients Claims
analysis suggests incidence of hospital-treated aSAH may be as high as ~70K Addressable Patients ~50% of patients who survive the initial month remain permanently dependent on a caregiver to maintain daily living Hospitalization charges
can be up to ~$530k for an aSAH patient aSAH is among the most highly reimbursed Diagnosis-Related Groups (DRGs) in neuro ICU 70% of aSAH Cases Result in Death or Permanent Disability Most Critical Unmet Needs Sources: ClearView Analysis
(2025). Forian Claims Data. Becske T. (2018). Steven (2020). Hoh (2023). Etminan. JAMA Neurol. 2019; Fegin. The Lancet Neurology. 2009; Labovitz. Neuroepidemiology. 2006; Shea. Neurosurgery. 2007, Linn. Stroke. 1996; Anderson. Stroke. 2000;
Daniere. J de Radiologie Diagnostique. 2015; Ingall. Stroke. 1989; Giordan et al. J Neurosurg. 2021; Rinkel et al. Lancet Neurol. 2011; Intl Study of Unruptured Intracranial Aneurysms Investigators. NEJM. 1998.
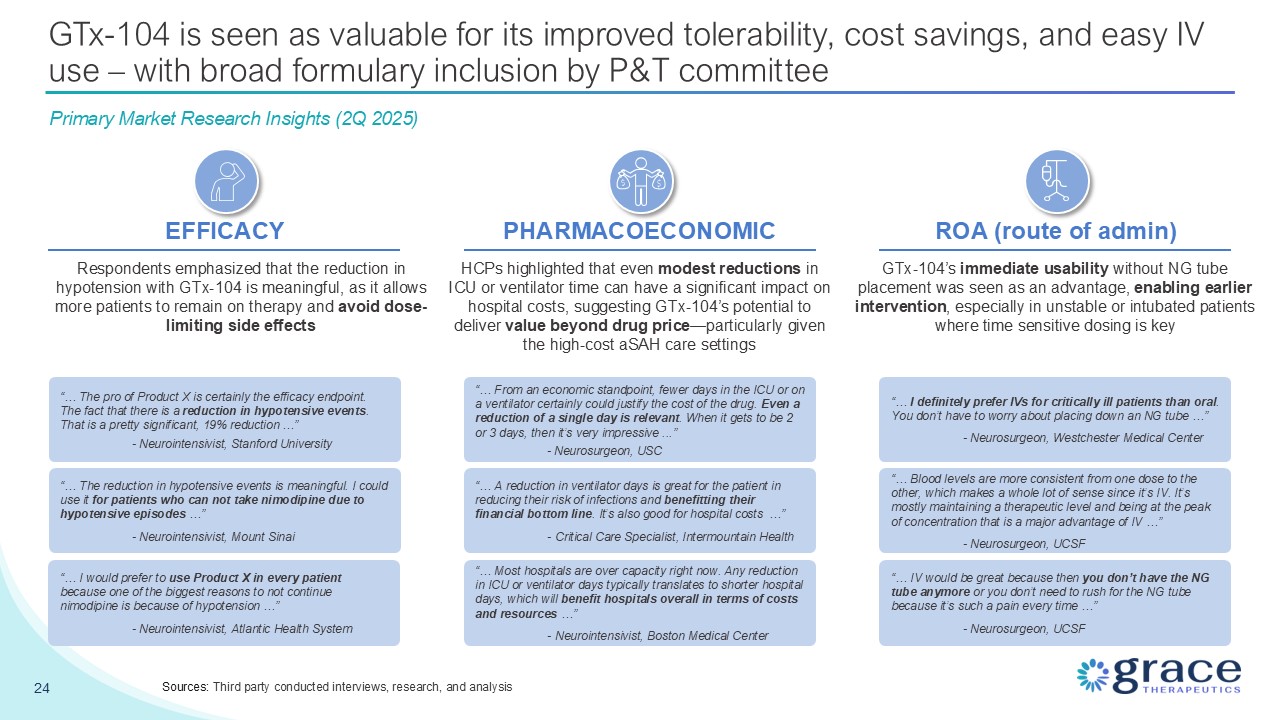
24 GTx-104 is seen as valuable for its improved tolerability, cost savings, and
easy IV use – with broad formulary inclusion by P&T committee EFFICACY PHARMACOECONOMIC ROA (route of admin) “… The pro of Product X is certainly the efficacy endpoint. The fact that there is a reduction in hypotensive events. That is
a pretty significant, 19% reduction …” - Neurointensivist, Stanford University “… The reduction in hypotensive events is meaningful. I could use it for patients who can not take nimodipine due to hypotensive episodes …” -
Neurointensivist, Mount Sinai “… I would prefer to use Product X in every patient because one of the biggest reasons to not continue nimodipine is because of hypotension …” - Neurointensivist, Atlantic Health System Respondents emphasized
that the reduction in hypotension with GTx-104 is meaningful, as it allows more patients to remain on therapy and avoid dose-limiting side effects HCPs highlighted that even modest reductions in ICU or ventilator time can have a significant
impact on hospital costs, suggesting GTx-104’s potential to deliver value beyond drug price—particularly given the high-cost aSAH care settings GTx-104’s immediate usability without NG tube placement was seen as an advantage, enabling
earlier intervention, especially in unstable or intubated patients where time sensitive dosing is key “… From an economic standpoint, fewer days in the ICU or on a ventilator certainly could justify the cost of the drug. Even a reduction of
a single day is relevant. When it gets to be 2 or 3 days, then it’s very impressive ...” - Neurosurgeon, USC “… A reduction in ventilator days is great for the patient in reducing their risk of infections and benefitting their financial
bottom line. It’s also good for hospital costs …” - Critical Care Specialist, Intermountain Health “… Most hospitals are over capacity right now. Any reduction in ICU or ventilator days typically translates to shorter hospital days, which
will benefit hospitals overall in terms of costs and resources …” - Neurointensivist, Boston Medical Center “… I definitely prefer IVs for critically ill patients than oral. You don’t have to worry about placing down an NG tube …” -
Neurosurgeon, Westchester Medical Center “… Blood levels are more consistent from one dose to the other, which makes a whole lot of sense since it’s IV. It’s mostly maintaining a therapeutic level and being at the peak of concentration that
is a major advantage of IV …” - Neurosurgeon, UCSF “… IV would be great because then you don’t have the NG tube anymore or you don’t need to rush for the NG tube because it’s such a pain every time …” - Neurosurgeon, UCSF Primary Market
Research Insights (2Q 2025) Sources: Third party conducted interviews, research, and analysis

Concentration of aSAH Care – Efficient Commercialization 25 aSAH-Treating
Institutions Concentration PatientVolume Analysis includes n = 3,227 institutions where at least one aneurysmal SAH patient is treated Concentration of aSAH Patients % of Institutions % of Patients ~80% of aSAH patients spread across
880 centers ~15 rep sales force to reach ~50% of aSAH patients, concentrated in 242 centers 1 Assumes each sales rep manages ~15 accounts. Sources: ClearView Analysis (2025). Forian Claims Data.
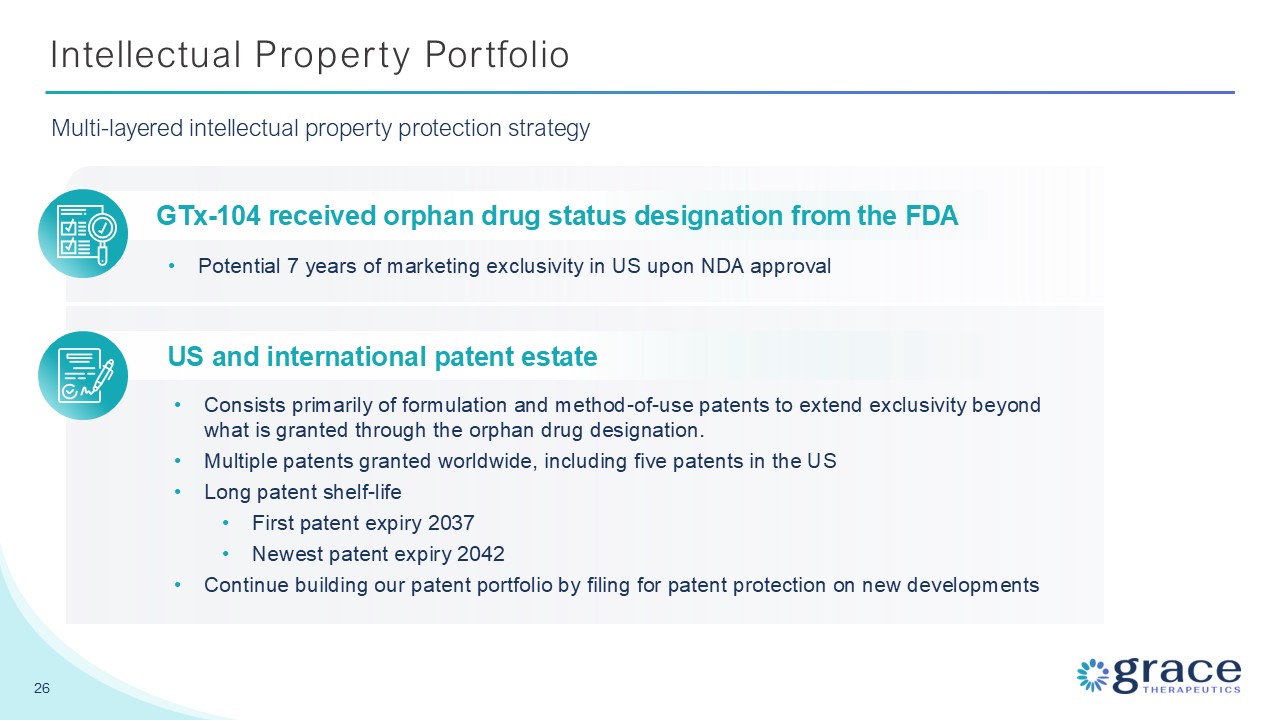
Intellectual Property Portfolio 26 Multi-layered intellectual property
protection strategy GTx-104 received orphan drug status designation from the FDA Potential 7 years of marketing exclusivity in US upon NDA approval US and international patent estate Consists primarily of formulation and method-of-use
patents to extend exclusivity beyond what is granted through the orphan drug designation. Multiple patents granted worldwide, including five patents in the US Long patent shelf-life First patent expiry 2037 Newest patent expiry
2042 Continue building our patent portfolio by filing for patent protection on new developments
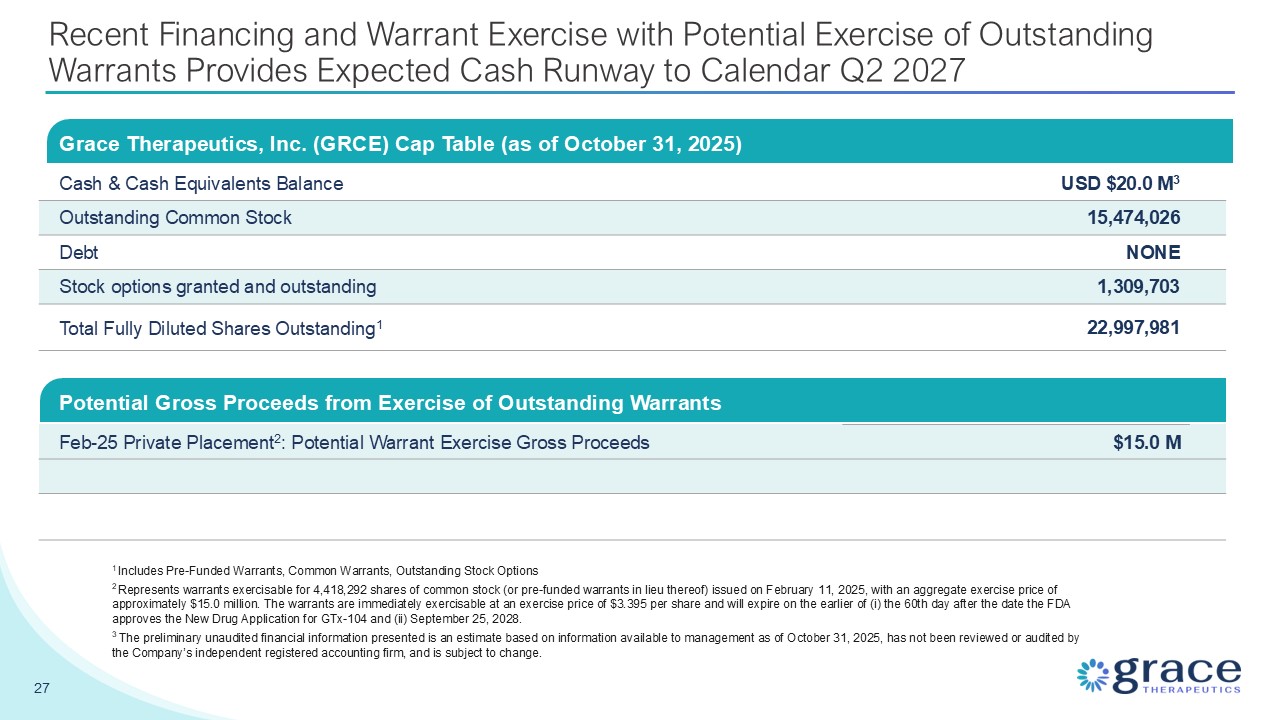
Recent Financing and Warrant Exercise with Potential Exercise of Outstanding
Warrants Provides Expected Cash Runway to Calendar Q2 2027 Grace Therapeutics, Inc. (GRCE) Cap Table (as of October 31, 2025) Cash & Cash Equivalents Balance USD $20.0 M3 Outstanding Common Stock 15,474,026 Debt NONE Stock
options granted and outstanding 1,309,703 Total Fully Diluted Shares Outstanding1 22,997,981 27 1 Includes Pre-Funded Warrants, Common Warrants, Outstanding Stock Options 2 Represents warrants exercisable for 4,418,292 shares of common
stock (or pre-funded warrants in lieu thereof) issued on February 11, 2025, with an aggregate exercise price of approximately $15.0 million. The warrants are immediately exercisable at an exercise price of $3.395 per share and will expire on
the earlier of (i) the 60th day after the date the FDA approves the New Drug Application for GTx-104 and (ii) September 25, 2028. 3 The preliminary unaudited financial information presented is an estimate based on information available to
management as of October 31, 2025, has not been reviewed or audited by the Company’s independent registered accounting firm, and is subject to change. Potential Gross Proceeds from Exercise of Outstanding Warrants Feb-25 Private Placement2:
Potential Warrant Exercise Gross Proceeds $15.0 M

Experienced Leadership Team 28 Carrie D'Andrea VP Clinical Operations Loch
Macdonald, MD, PhD Chief Medical Officer Prashant Kohli Chief Executive Officer Amresh Kumar, PhD VP Program Management Robert J. DelAversano Principal Financial Officer and Principal Accounting Officer Alejandro A. Rabinstein,
MD Alex Choi, MD Andrew Ducruet, MD Sherry H-Y Chou, MD W. Taylor Kimberly, MD, PhD Management Team Scientific Advisory Board Deep aSAH Expertise in Research, Commercial, Drug & AHA Care Guidelines Development

Appendix (Deprioritized Programs)
30 GTx-102 Program Overview & Regulatory Update GTx-102 Novel oral spray
formulation of betamethasone intended to improve neurological symptoms of A-T patients Proof of concept supported by well-controlled Phase 1 trial with A-T patients PK bridging study topline results announced on 12/18/22 met all outcome
measures Sources: Fletcher Spaght market research; National Organization for Rare Disorders (NORD); Lefton-Greif (2000); U.S. National Cancer Institute, A-T (2015). Unmet Need (No drugs approved) Treatment primarily directed toward
control of symptoms Limited to speech, occupational and physical therapy Less than 20% of patients on any type of drug therapy for A-T symptoms Ataxia-Telangiectasia Complex genetic neurodegenerative disorder diagnosed during
infancy Inherited as an autosomal recessive trait, often affects more than one child in a family Average lifespan ~25 years Potential addressable market ~$150 million Regulatory FDA’s written responses to EoP1 provides feedback on
design of a single pivotal efficacy trial to support NDA Guidance includes primary endpoint scale and appropriate confirmatory evidence Plan to discuss with SAB potential trial design
31 GTx-101 Program Overview GTx-101 Non-narcotic, topical, bio-adhesive,
transparent film-forming bupivacaine spray Biphasic drug release expected to provide immediate and continuous relief Potential Addressable market ~$200m (PHN) to ~$2.5b (lidocaine patch replacement) PHN: Postherpetic Neuralgia Sources:
Fletcher Spaght, Inc. analysis (2022); CDC MMWR June 6, 2008. UK and several US states have reclassified gabapentin as a scheduled drug Unmet Need Oral therapies (gabapentin, anticonvulsants, opioids) can have side effects and insufficient
to manage pain in many cases Can be prone to abuse Lidocaine patches are hard to place, can cause skin irritation, are 12-hour on / off ~40% experience insufficient pain relief Postherpetic Neuralgia (rare disease) Caused by nerve
damage from the herpes zoster virus which causes shingles Burning, painful, itchy, loss of feeling, sensitivity to touch or temperature, feeling worn out Symptoms can last for several years or may be permanent Regulatory Completed
Phase 1 (single dose) in 2022 Met all primary outcome measures Clinical roadmap includes Phase 1 (multiple ascending dose) and Phase 2 (POC)