10-Q: Quarterly report pursuant to Section 13 or 15(d)
Published on November 10, 2021
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________
FORM
______________________________________
(Mark One)
For the quarterly period ended
or
For the transition period from to
Commission file number:
______________________________________
Acasti Pharma Inc.
(Exact name of registrant as specified in its charter)
______________________________________
| Québec, |
|
| (State or other jurisdiction of |
(I.R.S. Employer |
(Address of principal executive offices, including zip code)
(Registrant’s telephone number, including area code)
______________________________________
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| |
|
|
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
||
| |
☒ |
Smaller reporting company |
|
||
| Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities Exchange Act of 1934). Yes
The number of outstanding common shares of the registrant, no par value per share, as of November 10, 2021, was
ACASTI PHARMA INC.
QUARTERLY REPORT ON FORM 10-Q
For the Quarter Ended September 30, 2021
Table of Contents
| Page |
||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This quarterly report contains information that may be forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and forward-looking information within the meaning of Canadian securities laws, both of which we refer to in this quarterly report as forward-looking statements. Forward-looking statements can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking statements in this quarterly report include, among other things, information or statements about:
| • |
our ability to build a premier, late-stage specialty pharmaceutical company specialized in rare and orphan disease and focused on developing and commercializing products that improve clinical outcomes using novel drug delivery technologies; |
|
| • |
our ability to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, and more convenient drug delivery that can result in increased patient compliance; |
|
| • |
the potential for our drug candidates to receive orphan drug status from the U.S. Food and Drug Administration (“FDA”) or regulatory approval under the Section 505 (b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act; |
|
| • |
the future prospects of our GTX-104 drug candidate, including but not limited to GTX-104’s potential to be administered to improve the management of hypotension; GTX-104’s potential to reduce the incidence of vasospasm and lead to better outcomes; the ability of GTX-104 to achieve a PKpharmacokinetic (“PK”) and safety profile similar to the oral form of nimodipine; GTX-104’s potential to provide improved bioavailability and the potential for reduced use of rescue therapies, such as vasopressors; the timing of the completion of the PK bridging study, and the timing and outcome of the Phase 3 safety study for GTX-104; our ability to ultimately file a new drug application (“NDA”) for GTX-104 under Section 505 (b)(2) NDA; and the timing and ability to receive FDA approval for marketing GTX-104; | |
| • |
the future prospects of our GTX-101 drug candidate, including but not limited to GTX-101’s potential to be administered to postherpetic neuralgia patients to treat pain associated with the disease; assumptions about the biphasic delivery mechanism of GTX-101, including its potential for rapid onset and continuous pain relief for up to eight hours; and the timing and outcomes of single ascending dose/multiple ascending dose and PK bridging studies and a Phase 2 and Phase 3 efficacy and safety study; the timing of an NDA filing under Section 505 (b)(2) for GTX-101; and the timing and ability to receive FDA approval for marketing GTX-101; | |
| • |
the future prospects of our GTX-102 drug candidate, including but not limited to GTX-102’s potential to provide clinical benefits to decrease Ataxia Telangiectasia symptoms; GTX-102’s potential ease of drug administration; the timing and outcomes of a PK bridging study and a Phase 3 efficacy and safety study for GTX-102; the timing of an NDA filing under Section 505 (b)(2) in connection with GTX-102; and the ability to receive FDA approval for marketing GTX-102; | |
| • |
the quality of our clinical data, the cost and size of our development programs, expectations and forecasts related to our target markets and the size of our target markets; the cost and size of our commercial infrastructure and manufacturing needs in the United States, European Union, and the rest of the world; and our expected use of a range of third-party contract research organizations (“CROs”) and contract manufacturing organizations (“CMOs”) at diverse locations; |
|
| • |
expectations and forecasts related to our intellectual property portfolio, including but not limited to consequences of orphan drug status designation from the FDA for our leading pipeline products; our patent portfolio strategy; and outcomes of our patent protection filings; |
| • |
our strategy, future operations, prospects and the plans of our management with a goal to enhance shareholder value, following our recent merger with Grace Therapeutics Inc. (“Grace”); |
|
| • |
our intellectual property position and duration of our patent rights; |
|
| • |
the potential adverse effects that the COVID-19 pandemic may have on our business and operations; |
|
| • |
our need for additional financing, and our estimates regarding our operating runway and future financing and capital requirements; |
|
| • |
our expectation regarding our financial performance, including our costs and expenses, liquidity, and capital resources; |
|
| • |
our projected capital requirements to fund our anticipated expenses; and |
|
| • | our ability to establish collaborations or obtain additional funding. |
Although the forward-looking statements in this quarterly report are based upon what we believe are reasonable assumptions, you should not place undue reliance on those forward-looking statements since actual results may vary materially from them. Important assumptions made by us when making forward-looking statements include, among other things, assumptions by us that:
| • | we are able to attract and retain key management and skilled personnel; | |
| • |
third parties provide their services to us on a timely and effective basis; |
|
| • |
we are able to take advantage of new business opportunities in the pharmaceutical industry; |
|
| • |
we are able to secure and defend our intellectual property rights, and to avoid infringing upon the intellectual property rights of third parties; |
|
| • |
the shareholder litigation relating to our merger with Grace is resolved in a manner favorable to us and we face no additional lawsuits or other proceedings, or any such matters, if they arise, are satisfactorily resolved; |
|
| • |
there are no material adverse changes in relevant laws or regulations; and |
|
| • |
we are able to obtain the additional capital and financing we require when we need it. |
In addition, the forward-looking statements in this quarterly report are subject to a number of known and unknown risks, uncertainties and other factors many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking statements, including, among others:
| • | We may not achieve our publicly announced milestones on time, or at all. | |
| • |
A failure to successfully integrate the businesses of Acasti and Acasti Pharma U.S., Inc. (“Acasti Pharma U.S.”, which is formerly Grace) in the expected timeframe would adversely affect our future results. |
|
| • |
Our future results will suffer if we do not effectively manage our expanded operations. |
|
| • |
We have incurred and expect to continue to incur substantial expenses related to the merger and expect to continue to incur substantial expenses related to the integration of the companies. |
|
| • | Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses. | |
| • | We may be subject to foreign exchange rate fluctuations. | |
| • | If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline. | |
| • | Lawsuits have been filed, and other lawsuits may be filed, against us and members of our board of directors challenging the Grace merger, and an adverse ruling in any such lawsuit may result in an award of damages against us. | |
| • |
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel. |
|
| • |
We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations and our ability to compete. |
|
| • |
We may face future product liability, and if claims are brought against us, we may incur substantial liability. |
|
| • |
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively. |
|
| • |
Even if our drug candidates receive regulatory approval in the United States, we may never obtain regulatory approval or successfully commercialize our products outside of the United States. |
|
| • |
We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable to our drug candidates, could hinder or prevent our drug candidates’ commercial success. |
|
| • |
Our commercial success depends upon attaining significant market acceptance of our drug products and drug candidates, if approved, among physicians, nurses, pharmacists, patients and the medical community. |
|
| • |
Guidelines and recommendations published by government agencies can reduce the use of our drug candidates and negatively impact our ability to gain market acceptance and market share. |
|
| • |
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug products, if approved, we may be unable to generate any revenue. |
|
| • |
If we obtain approval to commercialize any approved drug products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business. |
| • |
If we are unable to differentiate our drug products from branded reference drugs or existing generic therapies for similar treatments, or if the FDA or other applicable regulatory authorities approve products that compete with any of our drug products, our ability to successfully commercialize our drug products would be adversely affected. |
|
| • |
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively. |
|
| • |
We could incur substantial costs and disruption to our business and delays in the launch of our drug products if our competitors and/or collaborators bring legal actions against us, which could harm our business and operating results. |
|
| • |
The COVID-19 pandemic, or a similar pandemic, epidemic, or outbreak of an infectious disease, may materially and adversely affect our business and our financial results and could cause a disruption to the development of our drug candidates. |
|
| • |
We are subject to numerous complex regulatory requirements and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business. |
|
| • |
We are heavily dependent on the success of our lead drug candidates, GTX-104, GTX-102 and GTX-101. |
|
| • |
If the FDA does not conclude that our drug candidates satisfy the requirements for the 505(b)(2) regulatory approval pathway, or if the requirements for approval of any of our drug candidates under Section 505(b)(2) are not as we expect, the approval pathway for our drug candidates will likely take significantly longer, cost significantly more and encounter significantly greater complications and risks than anticipated, and in any case may not be successful. |
|
| • |
Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Failure can occur at any stage of clinical development. |
|
| • |
Delays in clinical trials are common and have many causes, and any delay could result in increased costs to us and could jeopardize or delay our ability to obtain regulatory approval and commence product sales. We may also find it difficult to enroll patients in our clinical trials, which could delay or prevent development of our drug candidates. |
|
| • |
Our drug products or drug candidates may cause adverse effects or have other properties that could delay or prevent their regulatory approval or limit the scope of any approved label or market acceptance, or result in significant negative consequences following marketing approval, if any. |
|
| • |
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our drug candidates, our business will be substantially harmed. |
|
| • |
An NDA submitted under Section 505(b)(2) subjects us to the risk that we may be subject to a patent infringement lawsuit that would delay or prevent the review or approval of our drug candidate. The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. |
|
| • |
Our business is subject to extensive regulatory requirements and our drug candidates that obtain regulatory approval will be subject to ongoing and continued regulatory review, which may result in significant expense and limit our ability to commercialize such products. |
| • |
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements. |
|
| • |
Any relationships with healthcare professionals, principal investigators, consultants, customers (actual and potential) and third-party payors are and will continue to be subject, directly, or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, marketing expenditure tracking and disclosure, or sunshine laws, government price reporting and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations. |
|
| • |
We are required to obtain regulatory approval for each of our drug candidates in each jurisdiction in which we intend to market such drug products, and the inability to obtain such approvals would limit our ability to realize their full market potential. |
|
| • |
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would have a material adverse effect on our business. |
|
| • |
Our success depends in part upon our ability to protect our intellectual property for our drug candidates, such as GTX-104, GTX-102 and GTX-101. |
|
| • |
Our drug development strategy relies heavily upon the 505(b)(2) regulatory pathway, which requires us to certify that we do not infringe upon third-party patents covering approved drugs. Such certifications often result in third-party claims of intellectual property infringement, the defense of which can be costly and time consuming, and an unfavorable outcome in any such litigation may prevent or delay our development and commercialization efforts, which would harm our business. | |
| • |
If we fail to comply with our obligations in the agreements under which we license rights to technology from third parties, or if the license agreements are terminated for other reasons, we could lose license rights that are important to our business. |
|
| • |
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties. |
|
| • |
We may be subject to claims challenging our inventorship or ownership of our patents and other intellectual property. |
|
| • |
Intellectual property rights do not necessarily address all potential threats to our competitive advantage. |
|
| • |
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect any of our other future drug products and drug candidates. |
|
| • |
We may not be able to protect our intellectual property rights throughout the world. |
|
| • |
We do not have internal manufacturing capabilities, and if we fail to develop and maintain supply relationships with various third-party manufacturers, we may be unable to develop or commercialize our drug candidates. |
|
| • |
Our contract manufacturers may encounter manufacturing failures that could delay the clinical development or regulatory approval of our drug candidates, or their commercial production if approved. |
| • |
We rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our drug candidates and our business could be substantially harmed. |
|
| • |
We rely on third parties to manufacture commercial and clinical supplies of our drug candidates, and we intend to rely on third parties to manufacture commercial supplies of any approved drug products. The commercialization of any of our drug products could be stopped, delayed, or made less profitable if those third parties fail to provide us with sufficient quantities of active pharmaceutical ingredients, excipients, or drug products, or fail to do so at acceptable quality levels or prices or fail to maintain or achieve satisfactory regulatory compliance. | |
| • |
The design, development, manufacture, supply, and distribution of our drug candidates are highly regulated and technically complex. |
|
| • |
We may not be successful in establishing development and commercialization collaborations which could adversely affect, and potentially prevent, our ability to develop our drug candidates. |
|
| • |
We may not be successful in maintaining development and commercialization collaborations, and any partner may not devote sufficient resources to the development or commercialization of our drug candidates or may otherwise fail in development or commercialization efforts, which could adversely affect our ability to develop certain of our drug candidates and our financial condition and operating results. |
|
| • |
We may be treated as a passive foreign investment corporation for U.S. federal income tax purposes. |
|
| • |
We may not be able to use our net operating loss carryforwards to offset future taxable income for Canadian or U.S. federal income tax purposes. |
|
| • |
We do not expect to pay any cash dividends for the foreseeable future. |
|
| • |
The price of our common shares may be volatile. |
|
| • |
Raising additional capital in the future may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or drug candidates. |
|
| • |
The market price of our common shares could decline as a result of operating results falling below the expectations of investors or fluctuations in operating results each quarter. |
|
| • |
An active market for our common shares may not be sustained. |
|
| • |
If we fail to meet applicable listing requirements, the NASDAQ Stock Market or the TSX Venture Exchange may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline. | |
| • |
We may pursue opportunities or transactions that adversely affect our business and financial condition. |
|
| • |
We are a “smaller reporting company” under the U.S. Securities and Exchange Commission’s (“SEC’s”) disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies. |
|
| • |
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act. |
|
| • |
We are a Québec incorporated company headquartered in Canada, and U.S. investors may be unable to enforce certain judgments against us. |
All of the forward-looking statements in this quarterly report are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition, or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this quarterly report.
We express all amounts in this quarterly report in U.S. dollars, except where otherwise indicated. References to “$” and “U.S.$” are to U.S. dollars and references to “C$” or “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this quarterly report to “Acasti,” “the Corporation,” “we,” “us” and “our” refer to Acasti Pharma Inc. and its consolidated subsidiaries, including Acasti Pharma U.S., which is formerly Grace.
Item 1: Financial Information
Unaudited Condensed Consolidated Interim Financial Statements
Condensed Consolidated Interim Financial Statements of
(Unaudited)
ACASTI PHARMA INC.
Three and Six-month periods ended September 30, 2021, and 2020
Condensed Consolidated Interim Balance sheet
(Unaudited)
| September 30, 2021 |
March 31, 2021 |
|||||||||||
| (Expressed in thousands of U.S. dollars except share data) |
Notes |
$ | $ | |||||||||
| Assets |
||||||||||||
| Current assets: |
||||||||||||
| Cash and cash equivalents |
||||||||||||
| Short-term investments |
5 | |||||||||||
| Receivables |
||||||||||||
| Assets held for sale |
7 | |||||||||||
| Prepaid expenses |
||||||||||||
| Total current assets |
||||||||||||
| Right of Use asset |
||||||||||||
| Intangible assets |
4 | |||||||||||
| Total assets |
||||||||||||
| Liabilities and shareholders’ equity |
||||||||||||
| Current liabilities: |
||||||||||||
| Trade and other payables |
||||||||||||
| Lease liability |
||||||||||||
| Total current liabilities |
||||||||||||
| Derivative warrant liabilities |
8 | |||||||||||
| Total liabilities |
||||||||||||
| Shareholders’ equity: |
||||||||||||
| Common shares |
|
4,9(a) | ||||||||||
| Additional paid-in capital |
||||||||||||
| Accumulated other comprehensive loss |
( |
) | ( |
) |
||||||||
| Accumulated deficit |
( |
) | ( |
) |
||||||||
| Total shareholder’s equity |
||||||||||||
| Commitments and contingencies |
14 | |||||||||||
| Total liabilities and shareholders’ equity |
||||||||||||
See accompanying notes to unaudited Interim financial statements.
Condensed Consolidated Interim Statements of Income (Loss) and Comprehensive Income (Loss)
(Unaudited)
Three-month and six-month periods ended September 30, 2021 and 2020
| Three-month periods ended |
Six-month periods ended |
|||||||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||||||
| (Expressed in thousands of U.S dollars, except per share data) |
Notes |
$ | $ | $ | $ | |||||||||||||||
| Operating expenses |
||||||||||||||||||||
| Research and development expenses, net of government assistance |
10 | ( |
) | ( |
) |
( |
) | ( |
) |
|||||||||||
| General and administrative expenses |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Sales and marketing expenses |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Impairment of intangible assets |
6 | ( |
) |
( |
) |
|||||||||||||||
| Impairment of equipment |
7 | ( |
) |
( |
) |
|||||||||||||||
| Loss from operating activities |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Financial income (expenses) |
11 | |||||||||||||||||||
| Net income (loss) and total comprehensive loss |
( |
) |
( |
) | ( |
) |
||||||||||||||
| Basic and diluted income (loss) per share |
( |
) |
( |
) | ( |
) |
||||||||||||||
| Weighted average number of shares outstanding |
||||||||||||||||||||
See accompanying notes to unaudited interim financial statements
Condensed Consolidated Interim Statements of Changes in Shareholder’s Equity
(Unaudited)
Three-month and six-month periods ended September 30, 2021 and 2020
| Common Shares |
||||||||||||||||||||||||||||
| (Expressed in thousands of U.S. dollars except share data) |
Notes |
Number |
Dollar |
Additional |
Accumulated |
Accumulated deficit |
Total |
|||||||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Balance, March 31, 2021 |
1 | ( |
) |
( |
) |
|||||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
- | - | - | - | ( |
) |
( |
) |
||||||||||||||||||||
| Cumulative translation adjustment |
- | - | - | - | ||||||||||||||||||||||||
| Stock based compensation |
12 | - | - | - | ||||||||||||||||||||||||
| Balance at June 30, 2021 |
( |
) |
( |
) |
||||||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
- | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
- | ( |
) | ( |
) | |||||||||||||||||||||||
| Stock based compensation |
12 | |||||||||||||||||||||||||||
| Common shares issued in relation to merger with Grace via share-for-share |
4 | |||||||||||||||||||||||||||
| Balance at September 30, 2021 |
( |
) | ( |
) | ||||||||||||||||||||||||
| Common Shares |
||||||||||||||||||||||||||||
| (Expressed in thousands of US dollars except for share data) |
Notes |
Number |
Dollar |
Additional paid-in capital |
Accumulated other comprehensive loss |
Accumulated deficit |
Total |
|||||||||||||||||||||
| $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Balance, March 31, 2020 |
( |
) |
( |
) |
||||||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
- | - | - | - | ( |
) |
( |
) |
||||||||||||||||||||
| Cumulative translation adjustment |
- | - | - | - | ||||||||||||||||||||||||
| Net proceeds from shares issued under the at-the-market (ATM) program |
|
9(a) | - | - | - | |||||||||||||||||||||||
| Stock based compensation |
12 | - | - | - | ||||||||||||||||||||||||
| Balance at June 30, 2020 |
( |
) |
( |
) |
||||||||||||||||||||||||
| Net loss and total comprehensive loss for the period |
- | ( |
) |
( |
) |
|||||||||||||||||||||||
| Cumulative translation adjustment |
- | |||||||||||||||||||||||||||
| Net proceeds from shares issued under the at-the-market (ATM) program |
|
9(a) | ||||||||||||||||||||||||||
| Stock based compensation |
( |
) |
( |
) |
||||||||||||||||||||||||
| Balance at September 30, 2020 |
( |
) |
( |
) |
||||||||||||||||||||||||
Condensed Consolidated Interim Statements of Cash Flows
(Unaudited)
Three-month and six-month periods ended September 30, 2021 and 2020
| Three-month periods ended |
Six-month periods ended |
|||||||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||||||
| (thousands of U.S. dollars) |
Notes |
$ | $ | $ | $ | |||||||||||||||
| Cash flows used in operating activities: |
||||||||||||||||||||
| Net income (loss) for the period |
( |
) |
( |
) | ( |
) |
||||||||||||||
| Adjustments: |
||||||||||||||||||||
| Amortization of intangible assets |
||||||||||||||||||||
| Depreciation of equipment |
||||||||||||||||||||
| Impairment of intangible assets |
||||||||||||||||||||
| Impairment of equipment |
||||||||||||||||||||
| Stock-based compensation |
12 | |||||||||||||||||||
| Change in fair value of warrant liabilities |
8 | ( |
) | ( |
) |
( |
) | ( |
) |
|||||||||||
| Write off of deferred financing costs of at-the-market (ATM) program |
9 | |||||||||||||||||||
| Unrealized foreign exchange (gain) loss |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Changes in non-cash working capital items |
13 | ( |
) | ( |
) |
( |
) | ( |
) |
|||||||||||
| Net cash used in operating activities |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Cash flows from (used in) investing activities: |
||||||||||||||||||||
| Acquisition of equipment |
( |
) |
( |
) |
||||||||||||||||
| Acquisition of short-term investments |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Maturity of short-term investment |
||||||||||||||||||||
| Net cash from (used in) investing activities |
( |
) |
( |
) | ( |
) |
||||||||||||||
| Cash flows from (used in) financing activities: |
||||||||||||||||||||
| Net proceeds from issuance of common shares under the at-the-market (ATM) |
|
9(a) | ||||||||||||||||||
| Deferred financing costs paid |
( |
) |
( |
) |
||||||||||||||||
| Net cash from (used in) financing activities |
||||||||||||||||||||
| Effect of exchange rate fluctuations on cash and cash equivalents |
( |
) |
( |
) | ( |
) |
||||||||||||||
| Translations effects on cash and cash equivalents related to reporting currency |
( |
) | ( |
) | ||||||||||||||||
| Net (decrease) increase in cash and cash equivalents |
( |
) | ( |
) |
( |
) | ( |
) |
||||||||||||
| Cash and cash equivalents, beginning of period |
||||||||||||||||||||
| Cash and cash equivalents, end of period |
||||||||||||||||||||
| Cash and cash equivalents are comprised of: |
||||||||||||||||||||
| Cash |
||||||||||||||||||||
| Cash equivalents |
||||||||||||||||||||
See accompanying notes to unaudited interim financial statements.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 1. |
Nature of Operation: |
Acasti Pharma Inc. (“Acasti” or the “Corporation”) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 3009 boul. de la Concorde East, Suite 102, Laval, Québec, Canada H7E 2B5.
In January 2020 and August 2020, the Corporation released Phase 3 clinical study results for the Corporation’s lead drug candidate, CaPre. The TRILOGY studies did not meet the primary endpoint which resulted in the Corporation’s Board of Directors making a decision not to proceed with a filing of an NDA with the FDA. With the completion of the TRILOGY studies beginning in the second half of fiscal 2021, marketing and research and development activities and expenses were reduced while management undertook a strategic review, and some CaPre related equipment and other assets were and continue to be classified as held for sale as they are expected to be sold.
In August 2021, the Corporation completed the acquisition of Grace Therapeutics, Inc. (“Grace”) a privately held emerging biopharmaceutical company focused on developing innovative drug delivery technologies for the treatment of rare and orphan diseases, via a share-for-share merger. The merged Corporation will focus on building a late-stage specialty pharmaceutical company specialized in rare and orphan diseases and focused on developing and commercializing products that improve clinical outcomes using novel drug delivery technologies. The Corporation seeks to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by the Corporation for further development may be already approved in the target indication or could be repurposed for use in new indications.
The Corporation has incurred operating losses and negative cash flows from operations in each year since its inception. The Corporation expects to incur significant expenses and continued operating losses for the foreseeable future. The Corporation expects its expenses will increase substantially in connection with its ongoing activities, particularly as it advances clinical development for the first three drug candidates in the Corporation’s pipeline; continue to engage contract manufacturing organizations (“CMOs”) to manufacture its clinical study materials and to develop large-scale manufacturing capabilities; seek regulatory approval for its product candidates; and add personnel to support its product development and future commercialization.
The Corporation does not expect to generate revenue from product sales unless and until it successfully completes drug development and obtains regulatory approval, which the Corporation expects will take several years and is subject to significant uncertainty. To date, the Corporation has financed its operations primarily through public offerings and private placements of its common shares, warrants and convertible debt and the proceeds from research tax credits. Until such time that the Corporation can generate significant revenue from product sales, if ever, it will require additional financing, which is expected to be sourced from a combination of public or private equity or debt financings or other non-dilutive sources, which may include fees from collaborations with third parties. Arrangements with collaborators or others may require the Corporation to relinquish certain rights related to its technologies or drug product candidates. Adequate additional financing may not be available to the Corporation on acceptable terms, or at all. The Corporation’s inability to raise capital as and when needed would have a negative impact on its financial condition and its ability to pursue its business strategy.
The Corporation remains subject to risks similar to other companies in the biopharmaceutical industry, including compliance with government regulations, protection of proprietary technology, dependence on third party contractors and consultants and potential product liability, among others.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 1. |
Nature of Operation (continued): |
Reverse Stock Split
On August 26, 2021, the shareholders of the Corporation approved a resolution to undertake a reverse split of the common stock within a range of 1-6 to 1-8 with such specific ratio to be approved by the Acasti Board. All references in these financial statements to number of common shares, warrants and options, price per share and weighted average number of shares outstanding prior to the reverse split have been adjusted to reflect the approved reverse stock split of 1-
| 2. |
Summary of significant accounting policies: |
Basis of presentation:
These unaudited Consolidated Interim Financial Statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and on a basis consistent with those accounting principles followed by the Corporation and disclosed in note 2 of its most recent Annual Consolidated Financial Statements, except as disclosed in note 3 – Recent accounting pronouncements and policies and note 4 Acquisition of Grace, and should be read in conjunction with such statements and Notes thereto.
Use of estimates:
The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities (note 8), stock-based compensation (note 12), assets held for sale (note 5), acquisition of Grace, valuation of intangibles (note 4) and the take-or-pay contract (note 14(a)). Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and developments expenditures at each reporting date, including whether contingencies should be accrued for, as well as in determining which research and development expenses qualify for investment tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and, therefore, could be different from the amounts recorded.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 2. |
Summary of significant accounting policies (continued): |
Intangible assets - acquired in-process research and development
In a business combination, the fair value of in-process research and development (“IPR&D”) acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as definite-lived intangible assets and amortized over the remaining useful life or discontinued. If discontinued, the intangible asset will be written off. Research and development (“R&D”) costs incurred after the acquisition are expensed as incurred.
The estimated fair values of identifiable intangible assets were determined using the "income approach" which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. Some of the assumptions inherent in the development of these asset valuations include the estimated net cash flows for each year for the asset (including net revenues, cost of products sold, R&D costs, and selling and marketing costs), the appropriate discount rate necessary to measure the risk inherent in each future cash flow stream, the life cycle of each asset, the potential regulatory and commercial success risk, competitive trends impacting the asset and each cash flow stream, as well as other factors.
Indefinite-lived assets are not amortized but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
The Corporation tests indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If the Corporation concludes it is more likely than not that the fair value is less than its carrying amount, a quantitative impairment test is performed. For its quantitative impairment tests, the Corporation uses an estimated future cash flow approach that requires significant judgment with respect to future sales volume, sales price and expense growth rates, changes in working capital use, the selection of an appropriate discount rate, asset groupings and other assumptions and estimates. The use of alternative estimates and assumptions could increase or decrease the estimated fair value of the assets and potentially result in different impacts to the Corporation's results of operations.
| 3. |
Recent accounting pronouncements |
In June 2016, the Financial Accounting Standards Board issued ASU 2016-13-Financial Instruments-Credit Losses (Topic 326), which amends guidance on reporting credit losses for assets held at amortized cost basis and available for sale debt securities. For assets held at amortized cost, the new guidance eliminates the probable initial recognition threshold in current U.S. GAAP and, instead, requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. ASU 2016-13 will affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, and any other financial assets not excluded from the scope that have the contractual right to receive cash. ASU 2016-13 is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2022. Management has not yet evaluated the impact of this ASU on the consolidated financial statements.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 4. |
Acquisition of Grace |
On August 27, 2021, the Corporation completed its acquisition of all outstanding equity interests in Grace Therapeutics Inc, via a merger. Grace, based in New Jersey and organized under the laws of Delaware, was a rare and orphan disease specialty pharmaceutical company.
In connection with the share-for-share noncash transaction, Grace was merged with a new wholly owned subsidiary of Acasti and became a subsidiary of Acasti. As a result, Acasti acquired Grace’s entire therapeutic pipeline consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of various granted and pending patents in various jurisdictions worldwide. Under the terms of the acquisition, each issued and outstanding share of Grace common stock was automatically converted into the right to receive Acasti common shares equal to the equity exchange ratio set forth in the merger agreement.
Consideration for acquisition
A total of
| Total common shares issued |
||||
| Acasti share price (closing share price on August 27, 2021) |
$ | |||
| Fair value of common shares issued |
$ |
The acquisition of Grace has been accounted for as a business combination using the acquisition method of accounting. This acquisition method requires, among other things, that assets acquired, and liabilities assumed in a business combination be recognized at their fair values as of the acquisition date. The valuation of assets acquired, and liabilities assumed has not yet been finalized as of September 30, 2021. As a result, the Corporation recorded preliminary estimates for the fair value of assets acquired and liabilities assumed as of the acquisition date. Finalization of the valuation during the measurement period could result in a change in the amounts recorded for the acquisition date of fair value of intangible assets, goodwill, property and equipment, and income taxes, among other items. The completion of the valuation will occur no later than one year from the acquisition date.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 4. |
Acquisition of Grace (continued): |
The following table summarizes the preliminary fair value of assets acquired and liabilities assumed as of the acquisition date:
| Assets acquired and liabilities assumed |
||||
| Cash and equivalents |
$ | |||
| Prepaid expenses and other current assets |
$ | |||
| Intangible assets – in-process research and development |
$ | |||
| Accounts payable and accrued expenses |
$ | ( |
) | |
| Total assets acquired and liabilities assumed |
$ |
Intangible assets of $
Acquisition-related expenses, which were comprised primarily of regulatory, financial advisory and legal fees, totaled $
Pro Forma Financial Information
The following table presents the unaudited pro forma combined results of Acasti and Grace for the six months ended September 30, 2021, as if the acquisition of Grace had occurred on April 1, 2020:
| Six months ended September 30, 2021 $ |
||||
| Net loss |
( |
) | ||
The unaudited pro forma condensed combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of Acasti and Grace. The unaudited pro forma financial information is not necessarily indicative of what the consolidated results of operations would have been had the acquisition been completed on April 1, 2020. In addition, the unaudited pro forma financial information is not a projection of future results of operations of the combined company, nor does it reflect the realization of any synergies or cost savings associated with the acquisition.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 5. |
Short-term investments |
The Corporation holds various marketable securities, with maturities greater than 3 months at the time of purchase, as follows:
| September 30, 2021 |
March 31, 2021 |
|||||||
| $ | $ | |||||||
| Term deposits issued in US currency earning interest at ranges between 0.19% and 0.30% and maturing on various dates from November 4, 2021, to December 20, 2021 |
||||||||
| Term deposits issued in CAD currency earning interest at ranges between 0.51% and 0.60% and maturing on various dates from December 2, 2021, to December 20, 2021 |
||||||||
| Total short-term investments |
||||||||
| 6. |
Impairment of intangible assets: |
In prior years, the Corporation entered into agreements with Neptune Wellness Solutions Inc. (Neptune) pursuant to which the Corporation obtained a license and exercised its option under this license agreement to pay in advance future royalties payable to Neptune. This license allowed the Corporation to exploit the intellectual property rights in order to conduct clinical trials for its CaPre drug candidate. The Corporation tests intangible assets for impairment should circumstances change or events occur that would indicate that the fair value of an asset may be below its carrying value. During the second quarter of fiscal 2021, the Corporation released its Phase 3 clinical programs data and its failure to meet its primary endpoints, and the resulting decision to not file an NDA to obtain FDA approval for CaPre. In addition, a significant share price reduction occurred. Due to these indicators of impairment under ASC 350, the Corporation undertook an analysis to determine the fair value of its intangible asset this quarter.
In assessing the magnitude of any impairment of the license the Corporation considered all available evidence, including (i) significant adverse impact from business climate due to the Phase 3 clinical program’s failure to meet its primary endpoints, and the resulting decision to not file an NDA to obtain FDA approval for CaPre, and the resulting internal forecasts that no cash flows from the use of the license was possible, and (ii) management’s estimate that a market place participant would place minimal to no value on the license if it were to be sold on its own or in combination with other assets, recognized or not, which is a level 3 measurement in the fair value hierarchy which included unobservable inputs. Accordingly, an impairment loss of $
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 7. |
Assets held for sale |
During the period, the Corporation determined to actively market for sale Other assets and Equipment and has met the criteria for classification of assets held for sale:
| September 30, 2021 |
March 31, 2021 |
|||||||
| $ | $ | |||||||
| Other assets (a) |
||||||||
| Equipment (b) |
||||||||
| a. |
Other assets |
Other assets represent krill oil (“RKO”) held by the Corporation that was expected to be used in commercial inventory scale up related to the development and commercialization of the CaPre drug candidate. Given that the development of CaPre will no longer be pursued by Acasti, the Corporation is expected to sell this reserve. The other asset is being recorded at the fair value less cost to sell. Management’s estimate of the fair value of the RKO less cost to sell was based primarily on estimated market prices at the end of the Corporation’s fiscal year ended March 31, 2021, obtained from an appraiser specializing in the krill oil market. Market prices have not changed materially since the end of the Corporation’s fiscal year ended March 31, 2021. These projections are based on Level 3 inputs of the fair value hierarchy and reflect management’s best estimate of market participants’ pricing of the assets as well as the general condition of the asset.
|
b.
|
Equipment |
| September 30, 2021 |
Cost, net of impairment |
Accumulated depreciation |
Net book value |
|||||||||
| $ | $ | $ | ||||||||||
| Furniture and office equipment |
( |
) | ||||||||||
| Computer equipment |
( |
) | ||||||||||
| Laboratory equipment |
( |
) | ||||||||||
| Production equipment |
( |
) | ||||||||||
| ( |
) | |||||||||||
Equipment is made up of laboratory, production, computer, and office equipment that was utilized in the development of CaPre. Similarly, to the intangible assets and Other assets, the announcement of the failed Phase 3 clinical trials for CaPre resulted in an impairment trigger for the laboratory and production equipment. The impairment loss is based on management’s estimate of the fair value of the equipment less cost to sell, which is based primarily on estimated market prices obtained from brokers specialized in selling used equipment. These projections are based on Level 3 inputs of the fair value hierarchy and reflect the Corporation’s best estimate of market participants’ pricing of the assets as well as the general condition of the assets.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 8. |
Derivative warrant liabilities: |
In connection with the Canadian public offering that closed on May 9, 2018, the Corporation issued a total of
In connection with the U.S. public offering that closed on December 27, 2017, the Corporation issued a total of
The derivative warrant liabilities are measured at fair value at each reporting period and the reconciliation of changes in fair value is presented in the following tables:
| Warrant liabilities issued |
Warrant liabilities issued |
||||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September30, 2021 |
September30, 2020 |
||||||||||||||
| Balance – beginning of period |
|||||||||||||||||
| Change in fair value |
( |
) | ( |
) | ( |
) | ( |
) | |||||||||
| Translation effect |
( |
) | ( |
) | |||||||||||||
| Balance – end of period |
|||||||||||||||||
| Fair value per share issuable |
|||||||||||||||||
The fair value of the derivative warrant liabilities was estimated using the Black-Scholes option pricing model and based on the following assumptions:
| Warrant liabilities issued |
Warrant liabilities issued December 27, 2017 |
|||||||||||||||
| September 30, 2021 |
March 31, 2021 |
September 30, 2021 |
March 31, 2021 |
|||||||||||||
| Exercise price |
CAD$ | CAD$ | USD$ | USD$ | ||||||||||||
| Share price |
CAD$ | CAD$ | USD$ | USD$ | ||||||||||||
| Risk-free interest |
% | % |
% | % | ||||||||||||
| Estimated life (years) |
||||||||||||||||
| Expected volatility |
% | % |
% | % | ||||||||||||
| Dividend |
nil |
nil |
nil | nil | ||||||||||||
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 9. |
Capital and other components of equity: |
| (a) |
“At-the-market” sales agreement: |
On February 14, 2019, the Corporation entered into an ATM sales agreement with B. Riley FBR, Inc. (“B. Riley”) pursuant to which common shares may be sold from time to time for aggregate gross proceeds of up to $
During the six-month period ended September 30, 2021,
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 9. |
Capital and other components of equity (continued): |
| (b) |
Warrants: |
The outstanding warrants of the Corporation are composed of the following as at September 30, 2021, and March 31, 2021:
| September 30, 2021 |
March 31, 2021 |
|||||||||||||||
| Number |
Amount |
Number |
Amount |
|||||||||||||
| Liability |
||||||||||||||||
| May 2018 Canadian public offering warrants(i) |
||||||||||||||||
| December 2017 U.S. public offering warrants(ii) |
||||||||||||||||
| Equity |
||||||||||||||||
| December 2017 US public offering broker warrants (iii) |
||||||||||||||||
| February 2017 Canadian public offering warrants (iv) |
||||||||||||||||
| (i) |
Warrants to acquire common share at an exercise price of CAD $ |
| (ii) |
Warrants to acquire common share at an exercise price of $ |
| (iii) |
Warrants to acquire common share at an exercise price of $ |
| (iv) |
Warrants to acquire common share at an exercise price of CAD expiring on February 21, 2022. |
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 10. |
Government assistance: |
Government assistance is comprised of a government grant from the Canadian federal government and research and development investment tax credits receivable from the Québec provincial government, which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. For the six-month periods ended September 30, 2021, and September 30, 2020, the Corporation recorded $
In September 2019, the Corporation was awarded up to CAD $
| 11. |
Net financial income: |
|
|
Three-month periods ended |
Six-month periods ended |
||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Foreign exchange gain (loss) |
( |
) |
||||||||||||||
| Write-off of deferred financing fees related to at-the-market (ATM) program |
( |
) |
( |
) | ||||||||||||
| Change in fair value of warrant liabilities |
||||||||||||||||
| Interest income |
||||||||||||||||
| Financial income |
|
|||||||||||||||
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 12. |
Stock-based compensation: |
At September 30, 2021, the Corporation has in place a stock option plan for directors, officers, employees, and consultants of the Corporation (“Stock Option Plan”). An amendment of the Stock Option Plan was approved by shareholders on August 26, 2021. The amendment provides for an increase to the existing limits for common shares reserved for issuance under the Stock Option Plan as well as certain changes to the minimum vesting period applicable to options granted to directors under the Stock Option Plan.
The Stock Option Plan continues to provide for the granting of options to purchase common shares. The exercise price of the stock options granted under this amended plan is not lower than the closing price of the common shares on the TSXV at the close of markets the day preceding the grant. The maximum number of common shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 10% of the aggregate number of issued and outstanding shares of the Corporation, from time to time. This resulted in an increase from
The total number of shares issued to any one consultant within any twelve-month period cannot exceed
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 12. |
Stock-based compensation (continued): |
The following table summarizes information about activities within the Stock Option Plan for the three and six-month periods ended:
| September 30, 2021 |
September 30, 2020 |
|||||||||||||||
| Weighted average |
Number of |
Weighted average |
Number of |
|||||||||||||
| CAD$ | CAD$ | |||||||||||||||
| Outstanding at beginning of period |
||||||||||||||||
| Granted |
||||||||||||||||
| Exercised |
||||||||||||||||
| Forfeited |
( |
) | ( |
) | ||||||||||||
| Expired |
||||||||||||||||
| Outstanding at end of period |
||||||||||||||||
| Exercisable at end of period |
||||||||||||||||
stock options were granted during the three and six-month periods ended September 30, 2021, and September 30, 2020. Compensation expense recognized under the Stock Option Plan for the three-month periods ended September 30, 2021, and September 30, 2020 was as follows:
| Three-month periods ended |
Six-month periods ended |
|||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||
| $ | $ | $ | $ | |||||||||
| Research and development expenses |
||||||||||||
| General and administrative expenses |
||||||||||||
| Sales and marketing expenses |
||||||||||||
Stock-based compensation payment transactions:
The fair value of stock-based compensation transactions is measured using the Black-Scholes option pricing model. Measurement inputs include share price on measurement date, exercise price of the instrument, expected volatility (based on weighted average historic volatility for a duration equal to the weighted average life of the instruments, life based on the average of the vesting and contractual periods for employee awards as minimal prior exercises of options in which to establish historical exercise experience; and contractual life for broker warrants), and the risk-free interest rate (based on government bonds). Service and performance conditions attached to the transactions, if any, are not taken into account in determining fair value. The expected life of the stock options is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility over a period similar to the life of the options is indicative of future trends, which may also not necessarily be the actual outcome.
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 13. |
Supplemental cash flow disclosure: |
| (a) |
Changes in non-cash operating items: |
| Three-month periods ended |
Six-month periods ended |
|||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||
| $ | $ | $ | $ | |||||||||
| Receivables |
( |
) | ( |
) |
( |
) | ( |
) |
||||
| Prepaid expenses |
( |
) | ( |
) | ||||||||
| Other assets |
||||||||||||
| Trade and other payables |
( |
) | ( |
) |
( |
) | ( |
) |
||||
| ( |
) | ( |
) |
( |
) | ( |
) |
|||||
| 14. |
Commitments and contingencies: |
In the ordinary course of business, the Corporation is at times subject to various legal proceedings and disputes. The Corporation assess its liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that the Corporation will incur a loss and the amount of the loss can be reasonably estimated, the Corporation records a liability in its consolidated financial statements. These legal contingencies may be adjusted to reflect any relevant developments. Where a loss is not probable or the amount of loss is not estimable, the Corporation does not accrue legal contingencies. While the outcome of legal proceedings is inherently uncertain, based on information currently available, management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on the Corporation’s financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to the Corporation’s financial position, results of operations, or cash flows. No reserves or liabilities have been accrued as at September 30, 2021.
| (a) |
Take or pay contract: |
On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antartic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre for a total value of $
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
Three-month and six-month periods ended September 30, 2021 and 2020
| 15. |
Subsequent events: |
“At-the-market” sales agreement:
On November 10, 2021, the Corporation filed a prospectus supplement relating to its at-the-market program with B. Riley, Oppenheimer& Co. Inc. and H.C. Wainwright & Co., LLC acting as agents. Under the terms of the ATM Sales Agreement and the prospectus supplement, the Corporation may issue and sell from time-to-time common shares having an aggregate offering price of up to $
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operation
This management’s discussion and analysis (“MD&A”), is presented in order to provide the reader with an overview of the financial results and changes to our balance sheet as at September 30, 2021, and for the three-month period then ended. This MD&A also explains the material variations in our results of operations, balance sheet and cash flows for the three and six-month periods ended September 30, 2021, and 2020.
Market data, and certain industry data and forecasts included in this MD&A, were obtained from internal corporation surveys and market research and those conducted by third parties hired by us, publicly available information, reports of governmental agencies and industry publications, and independent third-party surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of that information is not guaranteed. We have not independently verified any of the data from third-party sources or the underlying economic assumptions they have made. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s or contracted third parties’ knowledge of our industry, have not been independently verified. Our estimates involve risks and uncertainties, including assumptions that may prove not to be accurate, and these estimates and certain industry data are subject to change based on various factors, including those discussed in this quarterly report and in our most recently filed annual report on Form 10-K.
This MD&A, approved by the Board of Directors on November 10, 2021, should be read in conjunction with our unaudited condensed consolidated interim financial statements for the three and six-month periods ended September 30, 2021, and 2020 included in this quarterly report. Our interim financial statements were prepared in accordance with generally accepted accounting principles issued by the Financial Accounting Standards Board in the United States (“U.S. GAAP”).
All amounts appearing in this MD&A for the period-by-period discussions are in thousands of U.S. dollars, except share and per share amounts or unless otherwise indicated.
Business Overview
On August 27, 2021, we completed our acquisition of Grace via a merger following the approval of Acasti’s shareholders and Grace’s stockholders. Following completion of the merger, Grace became a wholly owned subsidiary of Acasti and was renamed Acasti Pharma U.S. Inc.
The successful completion of the merger positions Acasti to build a premier, late-stage specialty pharmaceutical company specialized in rare and orphan disease and focused on developing and commercializing products that improve clinical outcomes using novel drug delivery technologies. We seek to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, and more convenient drug delivery and increased patient compliance; all of which could result in improved patient outcomes. The active ingredients chosen by Acasti for further development may be already approved in a target indication or could be repurposed for use in new indications.
In connection with the merger, we acquired Grace’s entire therapeutic pipeline addressing critical unmet medical needs for the treatment of rare and orphan diseases, consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of more than 40 granted and pending patents in various jurisdictions worldwide. These drug candidates aim to improve clinical outcomes by applying proprietary formulation and drug delivery technologies to existing pharmaceutical compounds to achieve improvements over the current standard of care, or to provide treatment for diseases with no currently approved therapy.
Rare disorders represent an attractive area for drug development, and there remains an opportunity for Acasti to utilize already approved drugs that have established safety profiles and clinical experience to potentially address significant unmet medical needs. A key advantage of pursuing therapies for rare disorders is the potential to receive orphan drug designation (“ODD”) from the FDA. ODD provides for seven years of marketing exclusivity in the United States post-launch, provided certain conditions are met. Rare diseases also allow for more manageably scaled clinical trials and provide market opportunities that may require a smaller, more targeted commercial infrastructure.
In addition, the existing safety profiles of these products combined with the prospect to utilize the Section 505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act (the “FFDCA”) may provide a potentially shorter path to regulatory approval. Under Section 505(b)(2), if sufficient support of a product’s safety and efficacy either through previous FDA experience or sufficiently within the scientific literature can be established, it may eliminate the need to conduct some of the early studies that new drug candidates might otherwise require.
The specific diseases targeted for drug development by Acasti are well understood although these patient populations may remain poorly served by available therapies or in some cases approved therapies do not yet exist. We aim to effectively treat debilitating symptoms that result from these underlying diseases.
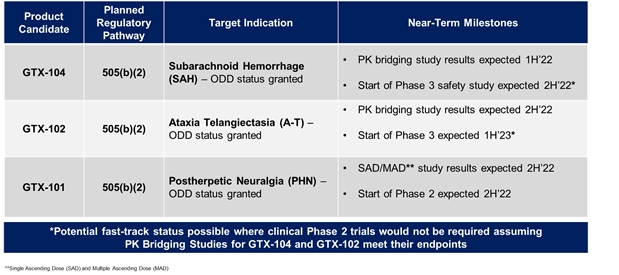
Our three most advanced programs are:
| • | GTX-104, an IV formulation of nimodipine designed to treat Subarachnoid Hemorrhage (“SAH”), a rare brain disorder for which Acasti Pharma U.S. (formerly Grace) had completed multiple pharmacokinetic (“PK”) studies. SAH is a central nervous system condition that causes acute bleeding in the brain and requires immediate medical attention to prevent long-term disability or death. GTX-104 could be administered to improve the management of hypotension and reduce the incidence of vasospasm in SAH patients and potentially lead to better outcomes. | ||
| • | GTX-102, an oral-mucosal betamethasone spray for the treatment of Ataxia Telangiectasia (“A-T”), an orphan pediatric complex genetic neurodegenerative disorder usually diagnosed in young children, for which no FDA approved treatment exists. | ||
| • |
GTX-101, a topical bioadhesive film-forming bupivacaine spray for Postherpetic Neuralgia (“PHN”), which is persistent and often causes debilitating pain following infection by the shingles virus. We believe that GTX-101 could be administered to patients with PHN to treat pain associated with the disease. |
Our management team possesses significant experience in drug delivery research and evaluation, clinical and pharmaceutical development and manufacturing, regulatory affairs, and business development, as well as being well versed in late-stage drug development and commercialization. The Acasti team has been collectively involved in the development and approval of several successful marketed drugs including TORADOL™, NAPROSYN™, ANDROGEL™, SUBSYS™, MARINOL™ and KEPPRA XR™.
The table below summarizes planned key calendar year milestones for our three clinical drug candidates:
GTX-104 Overview
Nimodipine was granted FDA approval in 1988, and is the only drug approved to improve neurological outcomes in SAH. It is only available in the United States as a generic oral capsule and as a branded oral liquid solution called NYMALIZE™, which is manufactured and sold by Arbor Pharmaceuticals. Nimodipine has poor water solubility and high permeability characteristics as a result of its high lipophilicity. Additionally, orally administered nimodipine has dose-limiting side-effects such as hypotension, low bioavailability resulting from high first-pass metabolism, and a narrow administration window as food effects lower bioavailability significantly. Nimodipine capsules are also difficult to administer, particularly to unconscious patients or those with impaired swallowing. Concomitant use with CYP3A inhibitors is contraindicated (NIMODIPINE Capsule PI).
NIMOTOP™ is an injectable form of nimodipine that is manufactured by Bayer Healthcare. It is approved in Europe and other regulated markets (but not in the United States), but it has limited utility for SAH patients because of its high organic solvent content, namely 23.7% ethanol and 17% polyethylene glycol 400 (NIMOTOP SmPC).
GTX-104 is a clinical stage, novel formulation of nimodipine for IV infusion in SAH patients. It uses surfactant micelles as the drug carrier to solubilize nimodipine. This unique nimodipine injectable formulation is composed of a nimodipine base, an effective amount of polysorbate 80, a non-ionic hydrophilic surfactant, and a pharmaceutically acceptable carrier for injection. GTX-104 is an aqueous solution substantially free of organic solvents, such that the nimodipine is contained in a concentrated injection solution, suspension, emulsion or complex as a micelle, a colloidal particle or an inclusion complex, and the formulation is stable and clear.
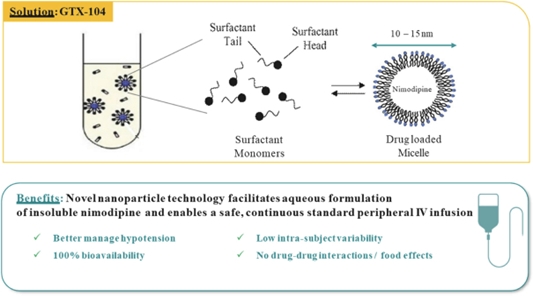
GTX-104 could provide a more convenient dosing schedule as it may be administered every twelve hours in patients with SAH as compared to generic nimodipine capsules or NYMALIZE™, which must be administered every four hours. In addition, since GTX-104 is peripherally infused, the dosing regimen is continuous during the period of therapy as compared to six times per day for both NYMALIZE™ oral solution and nimodipine oral capsules. Therefore, GTX-104 could be considered as a major contribution to patient care by potentially reducing the dosing frequency to twice daily. In addition, GTX-104 has the potential to provide improved bioavailability and lower intra-subject variability. Because of its IV formulation, we also expect it to reduce drug-drug interactions or food effects.
Despite the positive impact it has on recovery, physicians often must discontinue their patients on oral nimodipine, primarily as a result of hypotensive episodes that cannot be controlled by titrating the oral form of drug. Such discontinuation could potentially be avoided by administering GTX-104, which because of its IV administration, may obviate the complexity that results from the need for careful attention to the timing of nimodipine administration at least once before or two hours after a meal. Administration of GTX-104 via a peripheral vein is often much more comfortable for the patients compared to administration by central venous access, which can often be a difficult and invasive procedure. Also, unconscious patients will likely receive more consistent concentrations of nimodipine when delivered by the IV route as compared to oral gavage or a nasogastric tube. More consistent dosing is expected to result in a reduction of vasospasm and a better, more consistent management of hypotension. As summarized in the table below, we anticipate reduced use of rescue therapies, such as vasopressors, and expensive hospital resources, such as the angiography suite, by more effectively managing blood pressure with GTX-104. Reduced incidences of vasospasm could result in shorter length of stay and better outcomes.
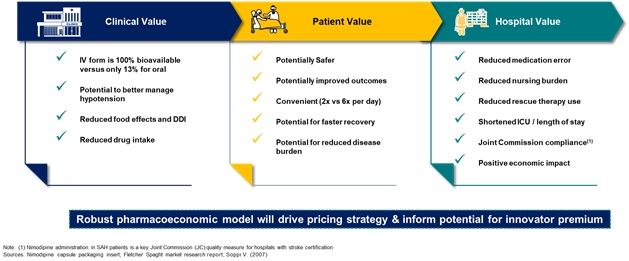
About Subarachnoid Hemorrhage (SAH)
SAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is rupture of an aneurysm. The result is a relatively uncommon type of stroke that accounts for about 5% of all strokes and has an incidence of six per 100,000 person years (Becske, 2018).
In contrast to common types of stroke in elderly individuals, an SAH often occurs at a relatively young age, with approximately half the affected patients younger than 60 years old (Becske, 2018). Particularly devastating for patients younger than 45, around 10% to 15% of aneurysmal SAH (“aSAH”) patients die before reaching the hospital (Rinkel, 2016), and those who survive the initial hours post hemorrhage are admitted or transferred to tertiary care centers with high risk of complications, including rebleeding and delayed cerebral ischemia (“DCI”). Systemic manifestations affecting cardiovascular, pulmonary, and renal function are common and often complicate management of DCI. Approximately 70% of aSAH patients experience death or a permanent dependence on family members, and half die within one month after the hemorrhage. Of those who survive the initial month, half remain permanently dependent on a caregiver to maintain daily living (Becske, 2018).
Treatment offerings currently include sustained hypervolemia, hemodilution, and/or induced hypertension (Triple-H therapy), calcium antagonists and angioplasty. Because vasospasm may result from an increase of calcium in the vascular smooth-muscle cell, a medical rationale has emerged for the use of calcium antagonists. The addition of calcium antagonists like nimodipine to the treatment arsenal for the prevention of cerebral vasospasm after aSAH is based on the notion that these drugs can counteract the influx of calcium into the vascular smooth-muscle cell (Rinkel, 2002).
The incidence of SAH in the United States is approximately 10 in every 100,000 persons per year (Becske, 2016; NINDS, 2016; Ingall, 1989; Schievink, 1995; Schievink, 1997; Zacharia, 2010), based on multiple analyses of the population of Rochester, Minnesota. Ingall (1989) studied the incidence of SAH in this population over the 40-year period from 1945 through 1984. At that time, the population of Rochester lent itself well to epidemiological studies because medical care was provided primarily by the Mayo Clinic. Over this period, the average annual incidence rate of aSAH remained constant at approximately 11 per 100,000 population. More recently, the American Heart Association/American Stroke Association Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage (Connolly, 2012) refer to the 2003 Nationwide Inpatient Sample as providing an annual estimate of 14.5 discharges for aSAH per 100,000 adults, although, because death resulting from aSAH often occurs before hospital admission (in an estimated 12% to 15% of cases), the true incidence may be higher. According to the U.S. Census Bureau, Population Estimates for 2015, the U.S. population was estimated at 321,418,820. Therefore, approximately 53,596 individuals experience aSAH each year. The total addressable market for SAH is approximately $300 million in the U.S., and an estimated 50,000 patients in the European Union based on annual inpatient admissions and the average length-of-stay.
GTX-104—R&D History and Clinical Studies to Date
During 2017 and 2018, Acasti Pharma U.S. (formerly Grace) evaluated GTX-104 in a four-part, single center, randomized, safety and dose-escalation and crossover study in over 80 healthy male and female subjects designed to assess the PK, bioavailability (“BA”), and the safety of GTX-104 administered via IV infusion compared to nimodipine oral capsules.
Details of the four-part PK study follow below:
| Part One: | ||
|
|
||
| Primary objective: |
Evaluate the preliminary cardiovascular safety and tolerability of incremental doses of IV GTX-104 in healthy male and female subjects |
|
| Method: |
Evaluate incremental dose-escalation of GTX-104 administered at dose levels of 0.3 mg/h to 1.22 mg/h over 16 hours, with dose-escalation occurring every 4 hours (0.3, 0.6, 0.9, and 1.22 mg/h) |
|
| Adverse Events: |
Arthralgia, constipation, flatulence, headache, infusion site irritation, peripheral edema, and vomiting—all adverse events (“AEs”) were rated as mild in severity |
|
| Part Two: | ||
|
|
||
| Primary Objective: |
Evaluate the PK and BA of GTX-104 administered via IV infusion compared to the reference product of oral nimodipine capsules and to select the dose of IV GTX-104 with an exposure profile most closely matching that of oral nimodipine capsules |
|
| Method: |
Two-period, crossover BA study. Pilot study that evaluated GTX-104 administered open-label as 1.22 mg/h continuous IV infusion for 16 hours compared to oral nimodipine (60 mg every 4 hours for 12 hours) in 12 subjects |
|
| Adverse Events: |
No serious adverse events ("SAEs") in any subjects. 20.0% of subjects reported non-serious AEs following administration of GTX-104 compared to 50.0% of subjects reporting AEs following administration of oral nimodipine |
|
| Part Three: |
||
| Primary Objective: |
Determine the comparative bioavailability of IV GTX-104 versus oral nimodipine capsules and to evaluate the safety and tolerability of IV GTX 104 compared to oral nimodipine capsules in healthy male and female subjects |
|
| Method: |
BA study, with GTX-104 administered as 1.1 mg/h continuous IV infusion for 28 hours compared to oral nimodipine capsules administered every four hours for 24 hours at a dose level of 60 mg in approximately 32 subjects |
|
|
|
||
| Adverse Events: |
No SAEs; 20.0% of the subjects reported non-serious AEs following administration of GTX-104 whereas 8 (50.0%) subjects reported AEs following administration of oral nimodipine. Fourteen (34.1%) subjects reported AEs following administration of GTX-104 whereas 18 (43.9%) subjects reported AEs following administration of oral nimodipine |
| Part Four: | ||
|
|
||
| Primary Objective: |
Determine the comparative BA of IV GTX-104 versus oral nimodipine capsules and to evaluate the safety and tolerability of IV GTX 104 compared to oral nimodipine capsules in healthy male and female subjects |
|
| Method: |
BA study: extension study with the same study design as Part Three, where only GTX-104 was administered open-label as a continuous IV infusion of 1.4 mg/h for 36 hours with oral nimodipine administered for 20 hours (approximately 24 subjects) |
|
| Adverse Events: |
No SAEs: 10 (41.7%) subjects reported AEs following administration of GTX-104 whereas eight (36.4%) subjects reported AEs following administration of oral nimodipine |
|
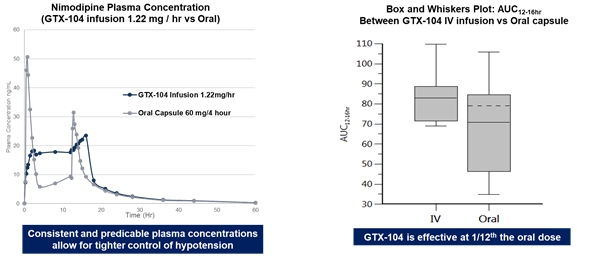
GTX-104 Near Term Milestones: - Conduct PK Bridging and Phase 3 Safety Studies
In September 2021, we initiated our planned PK bridging study to evaluate the relative bioavailability of GTX-104 compared to currently marketed oral nimodipine capsules in 50 healthy subjects. The PK study is the next required step in our proposed 505(b)2 regulatory pathway for GTX-104.
Results from this study are expected in the first half of calendar 2022, and after review with the FDA, will help determine the final design of our planned Phase 3 safety study of GTX-104 in SAH patients. If the PK study and related FDA review progress as planned, we expect to begin the Phase 3 safety study during the second half of 2022. If the safety study also meets its primary end points, we expect to submit the data in a Section 505(b)(2) NDA filing with the goal to obtain FDA approval.
GTX-102 Overview
GTX-102 is a novel, concentrated oral-mucosal spray of betamethasone intended to improve neurological symptoms of Ataxia Telangiectasia (“A-T”) for which there are no FDA approved therapies. GTX-102 is a stable, concentrated oral spray formulation comprised of the glucocorticoid betamethasone and other excipients that can be sprayed conveniently over the tongue of the A-T patient.
About Ataxia Telangiectasia
A-T is a rare genetic progressive autosomal recessive neurodegenerative disorder that affects children, with the hallmark symptoms of cerebellar ataxia and other motor dysfunction, and dilated blood vessels (telangiectasia) that occur in the sclera of the eyes. A-T is caused by mutations in the ataxia telangiectasia gene, which is responsible for modulating cellular response to stress, including breaks in the double strands of DNA.
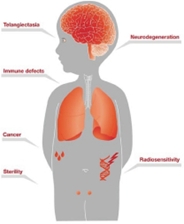
Children with A-T begin to experience balance and coordination problems when they begin to walk (toddler age), and ultimately become wheelchair-bound in their second decade of life. In pre-adolescence (between ages 5 and 8), patients experience oculomotor apraxia, dysarthria, and dysphagia. They also often develop compromised immune systems and are at increased risk of developing respiratory tract infections and cancer (typically lymphomas and leukemia) (U.S. National Cancer Institute A-T, 2015).
A-T is diagnosed through a combination of clinical assessment (especially neurologic and oculomotor deficits), laboratory analysis, and genetic testing. There is no known treatment to slow disease progression, and treatments that are used are strictly aimed at controlling the symptoms (e.g., physical, occupational or speech therapy for neurologic issues), or conditions secondary to the disease (e.g., antibiotics for lung infections, chemotherapy for cancer, etc.) (U.S. National Cancer Institute A-T, 2015). There are no FDA-approved therapeutic options currently available. Patients typically die by age 25 from complications of lung disease or cancer. According to a third-party report commissioned by Acasti Pharma US, A-T affects approximately 4,300 patients per year in the United States and has a potential total addressable market of $150 million, based on the number of treatable patients in the United States.
The U.S. National Institutes of Health (NIH) Genetics Home Reference, the U.S. National Organization for Rare Disorders (NORD), the U.S. National Cancer Institute, and the United States National Ataxia Foundation, all estimate the incidence of A-T worldwide to be between 1:40,000 and 1:100,000 live births. It has been reported in all races throughout the world and is represented equally in males and females (Lavin, 2007; Sedgwick and Boder, 1972).
For the purposes of estimating prevalence, the maximum survival age observed by Crawford et al., 40 years, has been used. Assuming a maximum survival of 40 years, the total number of A-T cases has been calculated from 1975 to 2015. The highest incidence rate reported in the United States of 1:40,000 has been used to obtain an estimate of A-T prevalence today. Between 1975 and 2015, the highest number of births in one year was 4,316,233 in 2007 (Martin, 2010; Martin, 2015) and so for the purposes of this prevalence calculation, this has been taken as the number of births per year.
Total A-T cases/year = 25 A-T births/million live births x 4.32 million live births/year = 108 A-T cases/year. Assuming that all 108 people possibly born with A-T are still alive today, the total number of individuals with A-T today in the United States, at the very outside estimate = 108 births/year x 40 years = 4320 cases. With a U.S. population of 321,251,852 (United States Census Bureau) the highest estimated prevalence of A-T is 4320:321,251,852 or 1:74,364.
GTX-102—R&D and Clinical Studies to Date
In a multicenter, double-blind, randomized, placebo-controlled crossover trial conducted in Italy, Zannolli et al. studied the effect of an oral liquid solution of betamethasone on the reduction of ataxia symptoms in 13 children (between ages 2 to 8 years) with A-T. Patients were randomly assigned to first receive either betamethasone or placebo at a dose of 0.1 mg/kg/day for 30 days: at full dose for the first 10 days, at a tapered dose on days 11–20 (i.e., for 4 days, 0.075 mg/kg/day; for 4 days, 0.050 mg/kg/day; and for 2 days, 0.025 mg/kg/day); and at full dose for the last 10 days (the full dose was tapered in the middle of the treatment phase to reduce risk from potential functional suppression of the hypothalamus-hypophysis-adrenal axis). Each phase of the trial was followed by a washout period of 30 days. The primary outcome measure was the reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”).
In the trial, oral liquid betamethasone reduced the ICARS total score by a median of 13 points in the intent-to-treat (“ITT”) population and 16 points in the per-protocol (“PP”) population (the median percent decreases of ataxia symptoms of 28% and 31%, respectively). In the ITT population, significant differences were observed in the posture and gait disturbance (p = 0.02), kinetic function (p = 0.02), and speech disorders ICARS subscales (p = 0.02), but not in the oculomotor disorders subscale (p > 0.05). Similar results were found in the PP population. Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require medical intervention. Small increases in body weight were observed in 12 patients on betamethasone and in 4 patients on placebo. Moon face was present in 8 patients on betamethasone. Clinical study results in A-T patients administered oral betamethasone indicated that betamethasone significantly reduced ICARS total score relative to placebo (P = 0.01). The median ICARS change score (change in score with betamethasone minus change in score with placebo) was -13 points (95% confidence interval for the difference in medians was -19 to -5.5 points).
| Clinical Study Results in A-T Patients Administered Oral Betamethasone |
||||||||||||||
| Placebo |
Betamethasone |
Efficacy |
||||||||||||
| ICARS |
Day -1 |
Day 31 |
Day -1 |
Day 31 |
Db |
95% Cl for the |
P valuec |
|||||||
| Total score |
46 (14-69) |
41.5 (26-68) |
50 (20-68) |
33 (19-55) |
-13 (-28 to 14) |
-19 to -5.5 |
.01 |
|||||||
| I. Posture and gait disturbance |
13.5 (3-30) |
14.5 (7-30) |
18 (7-29) |
9 (4-26) |
-5 (-15 to 5) |
-9.5 to -1.5 |
.02 |
|||||||
| II. Kinetic function |
22 (6-32) |
20.5 (13-31) |
23 (10-33) |
18 (8-28) |
-8 (-15 to 10) |
-10 to -0.5 |
.02 |
|||||||
| III. Speech disorder |
3 (1-5) |
2.5 (2-5) |
3 (2-5) |
2 (1-5) |
-1 (-3 to 1) |
-2.5 to -0.5 |
.02 |
|||||||
| IV. Oculomotor disorders |
3 (2-5) |
3.5 (1·5) |
3 (1-5) |
3 (1-5) |
0 (-2 to 2) |
-2 to 1 |
.43 |
|||||||
| a |
Data are medians (ranges). Thirteen ITT A-T patients are included. |
| b |
Median differences between the change in the ICARS score related to BETA treatment (d BETA) and the change related to placebo treatment (d placebo). |
| c |
P values calculated using the Wilcoxon rank sum test. |
Betamethasone significantly reduced ICARS total score relative to placebo (P = .01). The median ICARS change score (change in score with Betamethasone minus change in score with placebo) was -13 points (95% CI for the difference in medians was -19 to -5.5 points).
Based on the Zannolli data, we believe GTX-102 concentrated oral spray has the potential to provide clinical benefits in decreasing A-T symptoms, including assessments of posture and gait disturbance and kinetic, speech and oculomotor functions. In addition, GTX-102 may ease drug administration for patients experiencing A-T given its application of 1-3x 140µL of concentrated betamethasone liquid spray onto the tongue using a more convenient metered dose spray, as these A-T patients typically have difficulty swallowing (lefton-greif 2000).
GTX-102 PK Data to Date:
GTX-102 administered as a concentrated oral spray achieves similar blood levels at only 1/70th the volume of an oral solution of betamethasone. This is important for A-T patients who have difficulties swallowing large volumes of liquids.
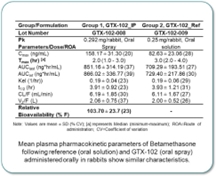
|
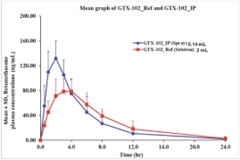
|
GTX-102 Near-Term Milestones: Conduct PK Bridging and Confirmatory Phase 3 Clinical Trials
Acasti Pharma US has licensed the data from the multicenter, double-blinded, randomized, placebo-controlled crossover trial from Azienda Ospedaliera Universitaria Senese, Siena, Italy, where Dr. Zannolli et. al. studied the effect of oral liquid solution of betamethasone to reduce ataxia symptoms in patients with A-T. Note that this oral liquid solution is not approved in the United States, and therefore is not available for clinical use. Betamethasone is only available in the United States as an injectable or as a topical cream. However, this license gives Acasti Pharma US the right to reference the study’s data in its NDA filing. On November 12, 2015, Acasti Pharma US submitted the data from the Zannolli study to the FDA’s Division of Neurology at a pre-Investigational New Drug (“IND”) meeting and received guidance from the agency on the regulatory requirements to seek approval.
Based on such FDA guidance, we plan to conduct a PK bridging study of our proprietary concentrated oral spray as compared to the oral liquid solution of betamethasone used in the Zannolli study. We believe this study may result in a better, more convenient use experience as patients with A-T often have trouble swallowing. Additionally, based on the FDA’s subsequent guidance and assuming the PK bridging study meets its primary endpoint, we plan to conduct a confirmatory Phase 3 safety and efficacy trial in A-T patients. If both studies meet their primary endpoints, an NDA filing under Section 505(b)(2) would follow.
GTX-101 Overview
GTX-101 is a topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (“PHN”). GTX-101’s metered-dose of bupivacaine spray forms a thin bioadhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches, we believe that the biphasic delivery mechanism of GTX-101 has the potential for rapid onset and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 study.
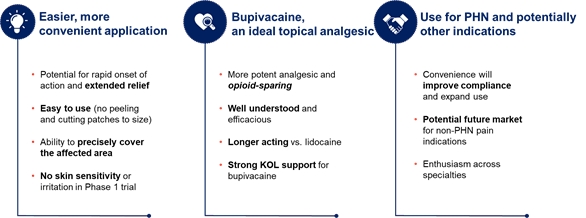
About Postherpetic Neuralgia (PHN)
PHN is neuropathic pain due to damage caused by the varicella zoster virus (“VZV”). Infection with the VZV causes two distinct clinical conditions. Primary VZV infection causes varicella (i.e., chickenpox), a contagious rash illness that typically occurs among young children. Secondary VZV can reactivate clinically, decades after initial infection, to cause herpes zoster (“HZ”), otherwise known as shingles. Acute HZ arises when dormant virus particles, persisting within an affected sensory ganglion from the earlier, primary infection with VZV become reactivated when cellular immunity to varicella decreases. Viral particles replicate and may spread to the dorsal root, into the dorsal horn of the spinal cord, and through peripheral sensory nerve fibers down to the level of the skin. Viral particles also may circulate in the blood. This reactivation is accompanied by inflammation of the skin, immune response, hemorrhage, and destruction of peripheral and central neurons and their fibers. Following such neural degeneration, distinct types of pathophysiological mechanisms involving both the central and peripheral nervous systems may give rise to the severe nerve pain associated with PHN.
While the rash associated with HZ typically heals within two to four weeks, the pain may persist for months or even years, and this PHN manifestation is the most common and debilitating complication of HZ. There is currently no consensus definition for PHN, but it has been suggested by the Centers for Disease Control and Prevention (“CDC”) that PHN is best defined as pain lasting at least three months after resolution of the rash. PHN persisting beyond three months may occur in 10% to 20% of HZ patients aged over fifty years (CDC Morbidity and Mortality Weekly Report, 2008).
PHN is associated with significant loss of function and reduced quality of life, particularly in the elderly. It has a detrimental effect on all aspects of patients’ quality of life. The nature of PHN pain varies from mild to excruciating in severity, constant, intermittent, or triggered by trivial stimuli. Approximately half of patients with PHN describe their pain as “horrible” or “excruciating,” ranging in duration from a few minutes to constant on a daily or almost daily basis (Katz, 2004). The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the quality of life and leading to social withdrawal and depression. PHN is the number-one cause of intractable, debilitating pain in the elderly, and has been cited as the leading cause of suicide in chronic pain patients over the age of 70 (Hess, 1990).
Current treatment of PHN most often consists of oral gabapentin and lidocaine patches, and refractory cases may be prescribed opioids to address persistent pain. Gabapentin and opioid abuse have continued to proliferate, and lidocaine patches are suboptimal for many reasons. According to a third-party report commissioned by Acasti Pharma US, approximately 40% of patients using lidocaine patches experience insufficient pain relief. Lidocaine patches are difficult to use, fall off, and look unsightly with possible skin sensitivity and irritation. Additionally, an optimal analgesic effect could take up to two weeks to be achieved. PHN affects approximately 150,000 patients per year in the United States. According to a third-party report commissioned by Acasti Pharma US, the total addressable market for GTX-101 is $1.6 billion, consisting of $400 million for PHN pain and $1.2 billion for non-PHN pain.
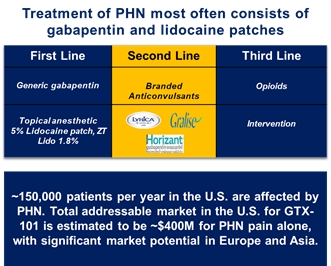
While the PHN will resolve within 1 to 2 months in many cases, and within the year in the majority of cases, it may persist in some patients for an extended period of time (more than 1 year), adding to the prevalence. On average, 4.6% of patients with PHN still experience pain at 1 year following development of the HZ rash, with 9% being the most reported. In a very small number of patients (2%), PHN remains persistent for over 5 years. Assuming in the worst case that 2% of PHN patients will experience pain for up to 10 years, an extra 2500 patients per year for 10 years could be added to the prevalence of 125,000 a year, adding 25,000 patients to any given year.
The CDC estimates that there are 1 million cases of HZ a year in the United States. The definition of PHN used in the pivotal study for the approved HZ vaccine was, “pain persisting or appearing more than 90 days after the onset of rash (Oxman, 2005).” Using this definition, and the numbers provided by the CDC, PHN would occur in approximately125,000-150,000 new cases per year.
GTX-101 R&D History and Clinical Studies Completed to Date
To date, Acasti Pharma US has conducted three Phase I studies in healthy volunteers to assess the PK, safety and tolerability of GTX-101 and to determine the plasma levels of bupivacaine HCl administered as a single dose in various concentrations, namely 30 mg (three sprays), 50 mg (five sprays), 70 mg (seven sprays) or 100 mg (ten sprays).
The initial study was conducted to determine the PK levels of GTX-101 following a single dose of either 30 mg, 50 mg or 70 mg, and to compare the plasma levels to those produced by a single 30 mg dose of injectable bupivacaine (SENSORCAINETM). In this study, the plasma levels of bupivacaine were below the limit of quantitation (limit of quantitation (“LOQ”) was 1.00 ng/mL) for almost all subjects administered GTX-101, and at almost all time points. Mean Cmax and AUC0-T for injectable bupivacaine were 129.3 ng/mL and 517.7 ng/mL, respectively. Bupivacaine was not detected due to assay sensitivity limited to 1ng/ml.
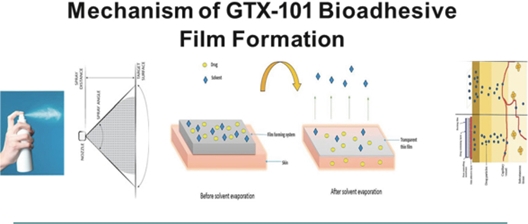
The second study investigated the PK, safety, and tolerability of a single 100 mg dose (ten sprays) of GTX-101. The mean bupivacaine Cmax in this study was 1.249 ng/mL for the first set of samples and 1.067 ng/mL for the second set of samples; the two mean values differing from each other by less than 20%. The LOQ of the bioanalytical method used for this study was 5 pg/mL. This study confirmed the Cmax values as being similar from two sets of samples collected from the same patients at the same time points.
In the third study, the PK, safety, and tolerability of a single 100 mg dose (ten sprays) of GTX-101 were again investigated. This study was a single-center, non-randomized, single dose, open-label, 1-period, 1-treatment design in 10 healthy male and female subjects. The PK results show the maximum observed plasma concentration of bupivacaine was reached within 20 to 48 hours for all subjects. The maximum concentration reached was 19.59 ng/mL. This study confirmed that bupivacaine delivered as a spray (GTX-101) is well absorbed through the skin, as demonstrated in the graph below.
In all three studies, the administration of GTX-101 to healthy volunteers was safe and well tolerated. In addition, no evidence of skin irritation was observed at the application site following the spray administrations.
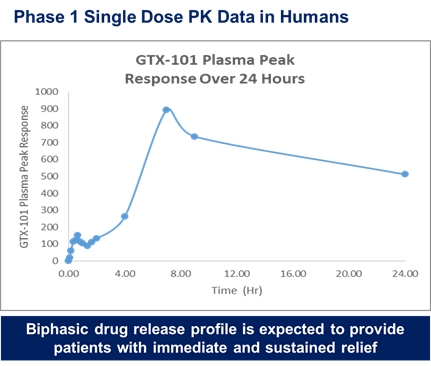
GTX-101 Near-Term Milestones: Conduct Dose Ranging Phase 1 Clinical Trials of GTX-101
We believe that the PHN pain market will continue to grow, and non-opioid products like GTX-101 that can relieve PHN pain more quickly and in a sustained manner by means of a more efficient delivery system, will be an attractive therapy option for patients and physicians. GTX-101 is administered by spraying a proprietary bupivacaine formulation over the affected area, which we believe has the potential to provide several advantages over currently marketed products such as the lidocaine patch, including faster onset of action, sustained pain relief, possibly lower dosing requirements and improved dosing convenience, all which could lead to increased patient compliance. The data from the single dose Phase 1 clinical trial for topical bupivacaine spray along with regulatory guidance from the FDA’s Division of Anesthesiology received at a pre-IND meeting on April 18, 2018 has informed the design of the preclinical toxicology, clinical and regulatory pathway to approval.
Overall Commercialization Strategy
We plan to retain our worldwide commercialization rights for some of our key drug candidates, while for other drug candidates we might consider collaboration opportunities to maximize market penetration and returns. If we receive regulatory approval, we expect to build a small and focused commercial organization in the United States to market and sell GTX-104 and GTX-102. We believe the patient populations and medical specialists for these indications are sufficiently concentrated to allow us to cost-effectively promote these drug products following approval for commercial sale. Given that GTX-101 will be targeted to a larger primary care and pain specialist market, if GTX-101 receives regulatory approval, it is likely we will seek commercial partnerships to fully exploit the market potential of this drug product.
As product candidates advance through the pipeline, our commercial plans may change. Clinical data, the size of the development programs, the size of the target market, the size of a commercial infrastructure and manufacturing needs may all have influence on U.S., European Union, and rest-of-world strategies.
Manufacturing and Supply
We currently do not own any manufacturing facilities. The manufacture of our pipeline of drug candidates is highly reliant on complex techniques and personnel aseptic techniques, which present significant challenges and require specialized expertise. Further, these processes undergo a high level of scrutiny by regulatory agencies. Consequently, we utilize a network of third-party contract manufacturers (“CMOs”) for manufacturing of our drug candidates. All CMOs are monitored and evaluated by us to assess compliance with regulatory requirements.
We work with and regularly inspect our manufacturers to review the manufacturing process for our drug candidates and to provide input on quality issues. We have addressed the risk of supply chain disruptions through risk management strategies designed to mitigate the effects of any disruptions. While this strategy creates additional effort and requires maintaining dialogue and traveling to and overseeing production at multiple facilities, we believe our manufacturing risks are better managed by utilizing a range of specialized third-party manufacturers at diverse locations.
Intellectual Property Portfolio
We have a strong and multi-layered intellectual property protection strategy, which we believe will create barriers to entry and solidify our position in the market. All leading pipeline products have received orphan status designation from the FDA, which could result in 7 years of marketing exclusivity in the United States and 10 years in Europe provided they receive the final marketing authorizations from the applicable government agencies, and they can meet the conditions for receiving such marketing exclusivity. In addition, we protect our drug candidates through a well-defined patent filing strategy. Our patent estate includes more than 40 granted and pending patents in various global jurisdictions, including 4 U.S. issued patents and 7 filed U.S. patent applications. We believe that our intellectual property portfolio, consisting primarily of composition and method-of-use patents, will protect the market value of our products by extending exclusivity beyond what is granted through the orphan designation. We intend to continue to build our patent portfolio by filing for patent protection on new developments with respect to our product candidates. We expect that these patents will, if and when issued, allow us to list our own patents in the Orange Book: Approved Drug Products with Therapeutic Equivalence issued by the FDA, to which potential competitors will be required to certify upon submission of their applications referencing our drug products, if approved.
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to the development of our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also rely on trade secrets relating to manufacturing know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen, and maintain our proprietary position. We may also rely on regulatory protections afforded through orphan drug status, data exclusivity, market exclusivity, and patent term extensions, where available.
We are actively seeking U.S. and international patent protection for a variety of technologies and intend to seek patent protection or rely upon trade secret rights to protect other technologies that may be used to discover and validate targets and that may be used to identify and develop novel pharmaceutical products. We seek these protections, in part, through confidentiality and proprietary information agreements.
Individual patents extend for varying periods depending on the date of filing or the date of issuance, and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than 5 years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. The actual protection afforded by a patent may vary on a product-by-product basis from country to country and can depend upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Acasti Pharma US has several issued U.S. patents and patent applications as well as patents and patent applications in other jurisdictions. Four patents for GTX-104 have been granted in the United States. One patent for GTX-101 has been granted in Europe, China, Hong Kong, Mexico and South Africa. One patent for GTX-102 has been granted in Japan.
Recent Developments
Management and Operations
Post-merger, Acasti continues to be led by Jan D’Alvise as President and CEO, and we continue to maintain our corporate headquarters in Laval, Quebec, Canada and a quality laboratory in Sherbrooke, Quebec, Canada. We will also maintain the Acasti Pharma U.S. research and development laboratory and commercial presence in North Brunswick, New Jersey.
About the Merger
In connection with our acquisition of Grace, Grace merged with Acasti Pharma US, a new wholly owned subsidiary of Acasti. Grace was the surviving company in the merger, was renamed Acasti Pharma US Inc., and is now a wholly owned subsidiary of Acasti. Grace stockholders received newly issued Acasti common shares pursuant to an equity exchange ratio formula set forth in the merger agreement.
The merger closed on August 27, 2021, following approval by Acasti shareholders at our annual and special meeting of shareholders, which took place on August 26, 2021.
Nasdaq Update
On May 11, 2021, we received written notice from the NASDAQ Listing Qualifications Department notifying us that based upon non-compliance with the $1.00 minimum bid price requirement set forth in NASDAQ Listing Rule 5550(a) as of May 10, 2021, our common shares were subject to delisting unless we timely requested a hearing before the NASDAQ Hearings Panel. We requested a hearing, which stayed any further action by NASDAQ pending the conclusion of the hearing process.
At the hearing on June 17, 2021, we presented a detailed plan of compliance for the NASDAQ Hearing Panel’s consideration, which included our commitment to implement a share consolidation if needed in connection with the merger with Grace to regain compliance with NASDAQ minimum bid price rule.
On July 12, 2021, the NASDAQ Hearings Panel issued its decision, which extended the time for us to regain compliance with Listing Rule 5550(a), subject to the following: 1) that we hold a shareholders meeting on or before August 26, 2021 to obtain approval for a share consolidation at a ratio allowing for long term compliance with Listing Rule 5550(a) and 2) on or before September 10, 2021, that we will have regained compliance with Listing Rule 5550(a).
On August 27, 2021, we announced that, in connection with the merger and in furtherance to the advisory resolution passed by Acasti shareholders at the shareholders meeting on August 26, 2021, approving a share consolidation, a share consolidation of our common shares at an 8-1 ratio had been implemented to regain compliance with NASDAQ’s Listing Rule 5550(a). The share consolidation became effective on August 31, 2021.
On September 22, 2021, Acasti announced the confirmation by NASDAQ that we had regained compliance with NASDAQ’s Listing Rule 5550(a).
Newly Elected Directors
As contemplated by the merger agreement, at the Acasti shareholders meeting on August 26, 2021, Vimal Kavuru and William Haseltine were elected as new directors (as well as our incumbent directors). Following the shareholders meeting, there remains one seat on our board of directors that is to be filled by a candidate recommended by the former Grace stockholders.
GHR Committee Composition and New Executive Titles
George Kottayil has been named Chief Operating Officer, U.S. of Acasti. Mr. Kottayil was a co-founder and previously served as Chief Executive Officer of Grace prior to its acquisition by Acasti. Pierre Lemieux continues as Chief Operating Officer, Canada, and Chief Scientific Officer of Acasti.
Mr. Vimal Kavuru, director, has replaced John Canan as a member of the Governance & Human Resources Committee of the Board. Mr. Canan will continue to serve as Chair of the Audit Committee of the Board.
COVID-19 Update
To date, the ongoing COVID-19 pandemic has not caused significant disruptions to our business operations and research and development activities.
The extent to which the COVID-19 pandemic impacts our business and prospects and the timing and completion of future clinical trials for our new drug candidates will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the COVID-19 pandemic and the actions to contain the COVID-19 pandemic or treat its impact, among others.
Basis of Presentation of the Financial Statements
Our Condensed Consolidated Interim Financial Statements, which include the accounts of our wholly owned subsidiaries, Acasti Innovations AG and Acasti Pharma U.S., have been prepared in accordance with U.S. GAAP, and the rules and regulations of the SEC related to interim reports filed on Form 10-Q. All intercompany transactions and balances are eliminated on consolidation.
Comparative Financial Information for the Three-Month and Six-Month Periods Ended September 30, 2021, and 2020
| Three-month periods ended |
Six-month periods ended |
|||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Net loss |
981 | (6,146 | ) |
(2,137 | ) | (10,812 | ) |
|||||||||
| Basic and diluted gain (loss) per share |
0.03 | (0.52 | ) |
(0.13 | ) | (0.93 | ) |
|||||||||
| Total assets |
119,796 | 13,832 | 119,796 | 13,832 | ||||||||||||
| Working capital1 |
50,069 | 9,962 | 50,069 | 9,962 | ||||||||||||
| Total non-current financial liabilities |
1,116 | 1,208 | 1,116 | 1,208 | ||||||||||||
| Total shareholders’ equity |
114,204 | 8,873 | 114,204 | 8,873 | ||||||||||||
1 Working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by U.S. GAAP requirements, the results may not be comparable to similar measurements presented by other public companies.
Statement of Net Loss
| Three-month periods ended |
Six-month periods ended |
|||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Research and development expenses |
(585 | ) |
(1,286 | ) |
(1,054 | ) | (3,042 | ) |
||||||||
| General and administrative expenses |
(2,957 | ) |
(1,324 | ) |
(5,633 | ) | (2,973 | ) |
||||||||
| Sales and marketing expenses |
(25 | ) | (134 | ) |
(25 | ) | (850 | ) |
||||||||
| Impairment of Intangible assets |
- | (3,706 | ) |
- | (3,706 | ) |
||||||||||
| Impairment of Equipment |
- | (1,584 | ) |
- | (1,584 | ) |
||||||||||
| Financial Income |
4,548 | 1,888 | 4,575 | 1,343 | ||||||||||||
| Net income (loss) |
981 | (6,146 | ) |
(2,137 | ) |
(10,812 | ) |
|||||||||
Results of Operations for the Three-Month and Six-month Periods Ended September 30, 2021, and 2020
The net income of $981 or $0.03 per share for the three months ended September 30, 2021, increased by $7,127 from the net loss of $6,146 or $0.52 per share for the three months ended September 30, 2020.
The reduction in net loss resulted primarily from a decrease in research and development expenses of $701. The TRILOGY Phase 3 clinical program for CaPre was completed and no significant R&D costs related to the acquired assets had yet been incurred since the close of the merger transaction on August 27, 2021. Sales and marketing expenses also decreased by $109 as we had not yet incurred expenses related to acquired assets. In addition, there was nil impairment loss recognized for the three months ended September 30, 2021, as compared to $5,290 impairment loss recognized in the three months period ended September 30, 2020, due to operational events for the TRILOGY clinical trials resulting in impairment triggers. These decreases are offset by an increase of $1,633 related to General and administrative expenses from the prior period, related to legal, tax, accounting and other professional fees incurred in relation to the merger.
Net financial income increased by $2,660 to a total gain of $4,548 for the three months ended September 30, 2021, as compared to net financial expenses of $1,888 for the three months ended September 30, 2020. This increase is due mostly to a decrease from the change in fair value of the derivative warrant liability as compared to the comparative fiscal quarter in 2020, caused by a proportionately higher decrease in our quarter over quarter closing share price, as well as an increase to foreign exchange gain of $1,111.
The net loss of $2,137 or $0.13 per share for the six months ended September 30, 2021, decreased by $8,675 from the net loss $10,812 or $0.93 per share for the six months ended September 30, 2020.
The decreased net loss resulted primarily from decreased R&D expenses of $1,054 for the six months ended September 30, 2021, as compared to R&D expenses of $3,042 for the six months ended September 30, 2020, as well as from increased financial income of $4,578 for the six months ended September 30, 2021, as compared to financial income of $1,343 for the six months ended September 30, 2020. The increase in financial income is due mostly to the change in fair value of the warrant derivative liability. General and administrative expenses increased from the comparative period related to legal, tax, accounting and other professional fees incurred in relation to the merger.
Sales and marketing expenses also decreased by $825 as a result of the discontinuation of CaPre commercialization activities.
Two separate derivative warrant liabilities are included in the statement of financial position as at September 30, 2021, and September 30, 2020. These derivative warrant liabilities stem from the financing transactions that took place in May 2018 and December 2017. The derivative warrant liabilities are re-measured to fair value at each reporting date using the Black-Scholes option pricing model. The valuations are mainly driven by the fluctuation in our share price resulting in an increased or decreased loss or gain related to the change in fair value of the warrant liabilities and increasing or decreasing the corresponding liability in the balance sheet.
Breakdown of Major Components of the Statement of Loss and Comprehensive Loss
| Research and development expenses |
||||||||||||||||
| Three Months Ended |
Six Months Ended |
|||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
332 | 286 | 626 | 720 | ||||||||||||
| Research contracts |
287 | 275 | 363 | 774 | ||||||||||||
| Professional fees |
27 | 201 | 43 | 355 | ||||||||||||
| Other |
30 | 55 | 63 | 114 | ||||||||||||
| Government grants & tax credits |
(129 | ) | (7 | ) | (129 | ) | (83 | ) |
||||||||
| Sub-total |
547 | 810 | 966 | 1,880 | ||||||||||||
| Stock-based compensation |
38 | 105 | 88 | 246 | ||||||||||||
| Depreciation and amortization |
- | 371 | - | 916 | ||||||||||||
| Total |
585 | 1,286 | 1,054 | 3,042 | ||||||||||||
| General and administrative expenses |
||||||||||||||||
| Three Months Ended |
Six Months Ended |
|||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
276 | 135 | 603 | 493 | ||||||||||||
| Professional fees |
2,203 | 753 | 4,144 | 1,455 | ||||||||||||
| Other |
402 | 247 | 707 | 486 | ||||||||||||
| Sub-total |
2,881 | 1,135 | 5,454 | 2,434 | ||||||||||||
| Stock-based compensation |
76 | 184 | 179 | 532 | ||||||||||||
| Depreciation |
- | 5 | - | 7 | ||||||||||||
| Total |
2,957 | 1,324 | 5,633 | 2,973 | ||||||||||||
| Sales and Marketing Expenses |
||||||||||||||||
| Three Months Ended |
Six Months Ended |
|||||||||||||||
| September 30, 2021 |
September 30, 2020 |
September 30, 2021 |
September 30, 2020 |
|||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Salaries and benefits |
25 | 112 | 25 | 502 | ||||||||||||
| Professional fees |
- | (23 | ) | - | 75 | |||||||||||
| Other |
- | (67 | ) | - | 18 | |||||||||||
| Sub-total |
25 | 22 | 25 | 595 | ||||||||||||
| Stock-based compensation |
- | 112 | - | 255 | ||||||||||||
| Total |
25 | 134 | 25 | 850 | ||||||||||||
During the three months ended September 30, 2021, we completed our previously announced acquisition of Grace and its assets via a merger. Research and development expenses before depreciation, amortization, and stock-based compensation expenses for the three months ended September 30, 2021, totaled $547 compared to $810 for the three months ended September 30, 2020.
The net decrease was mainly attributable to the reversal of prior period provision for tax credits after assessments and correspondence from tax authorities, of $122, as well as a reduction of professional fees of $174.
General and administrative expenses totaled $2,881 before depreciation and stock-based compensation expense for the three-months ended September 30, 2021 and increased by $1,746 from $1,135 for the three months ended September 30, 2020. This increase is a result of increased legal, tax, accounting and other professional fees related to the merger.
Sales and marketing expenses before stock-based compensation of $25 for the three months ended September 30, 2021, comparable to $22 before stock-based compensation expense for the three months ended September 30, 2021.
Stock-based compensation expense decreased by $287 for the three-month period ended September 30, 2021, amount to a loss of $114, as compared to a loss of $401 for the three-month period ended September 30, 2020. The decrease in expense is due to forfeited options as well as the fact that no options were granted in the three-month period ended September 30, 2021.
The depreciation and amortization expense decreased by $376 for the three-month period ended September 30, 2021, to nil as compared to $376 for the three-month period ended September 30, 2020. This is due to the impact of the equipment being classified as held for resale and no additional depreciation recognized.
Six-Months Ended September 30, 2021, compared to the Six-Months Ended September 30, 2020
During the six-months ended September 30, 2021, we completed our previously announced acquisition of Grace and in connection with the merger we acquired a pipeline of new drug candidates.
Research and development expenses before depreciation, amortization, and stock-based compensation expenses for the six months ended September 30, 2021, totaled $966 compared to $1,880 for the six months ended September 30, 2020. The net decrease was mainly attributable to the reduction in research professional fees as well as a reduction in headcount within the research and development departments associated with the completed TRILOGY trials and due to the reversal of prior period provision regarding uncertain tax positions after assessments and correspondence from tax authorities, for tax credits of $122.
General and administrative expenses totaled $5,454 before depreciation and stock-based compensation expense for the six-months ended September 30, 2021 and increased by $3,020 from $2,434 for the six months ended September 30, 2020. This increase was a result of increased legal, tax, accounting and other professional fees related to the merger.
Sales and marketing expenses were $25 for the six months ended September 30, 2021, compared to $595 before stock-based compensation expense for the six months ended September 30, 2021. The decrease was mainly due to decreased headcount resulting in salary and benefits decrease of $477.
Stock-based compensation expense decreased by $766 for the six-month period ended September 30, 2021, amount to a loss of $267, as compared to a loss of $1,003 for the six-month period ended September 30, 2020. The decrease in expense is due to forfeited options as well as the fact that no options were granted in the six-month period ended September 30, 2021.
The depreciation and amortization expense decreased by $923 for the six-month period ended September 30, 2021, to nil as compared to $923 for the six-month period ended September 30, 2020. This decrease was due to the impact of the equipment being classified as held for resale and no additional depreciation recognized.
Liquidity and Capital Resources
Share Capital Structure
Our authorized share capital consists of an unlimited number of Class A, Class B, Class C, Class D and Class E shares, without par value. Issued and outstanding fully paid shares, stock options, and warrants, were as follows for the periods ended (after giving effect to our 8:1 share consolidation, which became effective on August 31, 2021):
| September 30, 2021 Number outstanding |
March 31, 2021 Number outstanding |
|||||||
| Class A shares, voting, participating and without par value |
44,288,183 | 26,046,950 | ||||||
| Stock options granted and outstanding |
903,872 | 911,871 | ||||||
| May 2018 Canadian public offering of warrants exercisable at CAD$10.48 until May 9, 2023 |
824,218 | 824,218 | ||||||
| December 2017 U.S. public offering of warrants exercisable at US$10.08 until December 19, 2022 |
884,120 | 884,120 | ||||||
| December 2017 U.S. public offering broker warrants exercisable at US$10.10 until December 27, 2022 |
32,390 | 32,390 | ||||||
| February 2017 Canadian public offering of warrants exercisable at CAD$17.20 until February 21, 2022 |
215,491 | 215,491 | ||||||
| Total fully diluted shares |
47,148,278 | 28,915,040 | ||||||
Cash Flows and Financial Condition Between the three and Six-Months Ended September 30, 2021, and 2020
Summary
As at September 30, 2021, cash and cash equivalents totaled $36,929, a net increase of $25,377 compared to cash and cash equivalents totaling $11,552 at September 30, 2020.
Operating activities
During the three-months ended September 30, 2021, and September 30, 2020, our operating activities used cash of $6,104 and $4,178, respectively and during the six-months periods ended September 30, 2021, and September 30, 2020, our operating activities used cash of $9,505 and $8,345, respectively.
Investing activities
During the three-months ended September 30, 2021, our investing activities generated cash of $2,837, compared to cash used of $33 for the three-months ended September 30, 2020. The increase cash generated is a function in the increase in short term investments.
During the six-months ended September 30, 2021, our investing activities used cash of $4,090 compared to cash used of $69 for the six-months ended September 30, 2020. The decrease in cash generated is a function of the amounts of cash invested in short term investments.
Financing activities
During the three-months ended September 30, 2021, our financing activities provided cash totaling nil, compared to cash generated of $3,431, during the three months ended September 30, 2020, due to proceeds from the sale of shares under the ATM program.
During the six-months ended September 30, 2021, our financing activities provided cash totaling nil, compared to cash generated of $5,067, during the six-months ended September 30, 2020, due to proceeds from the sale of shares under the ATM program.
ATM program
On June 29, 2020, we entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley, Oppenheimer& Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend our ATM program. Under the terms of the Sales Agreement, which has a three-year term, we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. Subject to the terms and conditions of the Sales Agreement, the Agents will use their commercially reasonable efforts to sell the common shares from time to time, based upon our instructions. We have no obligation to sell any of the common shares and may at any time suspend sales under the Sales Agreement. We and the Agents may terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, we have provided the Agents with customary indemnification rights and the Agents will be entitled to compensation, at a commission rate equal to 3.0% of the gross proceeds from each sale of the common shares.
During the six-month period ended September 30, 2021, no common shares were sold under the ATM program. During the six-month period ended September 30, 2020, 835,386 common shares were sold for total net proceeds of approximately $5.2 million with commissions, legal expenses and costs related to the share sale amounting to $180. The common shares were sold at the prevailing market prices, which resulted in an average price of approximately $6.20 per share. Accordingly, proportional costs of $18 related to the common shares sold have been reclassified from deferred financings costs to equity.
On November 10, 2021, we filed a prospectus supplement relating to our ATM program to restore available capacity to $75,000,000, with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC continuing to act as Agents. Under the terms of the Sales Agreement and the prospectus supplement, we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. The common shares will be distributed at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The volume and timing of sales under the ATM program, if any, will be determined at the sole discretion of our board of directors and management.
Financial Position
The following table details the significant changes to the statements of financial position as at September 30, 2021, compared to the prior fiscal year end at March 31, 2021:
| Accounts |
Increase |
Comments |
||
| Cash and cash equivalents |
(14,013) |
See cash flow statement |
||
| Investments |
4,072 |
Increase in cash invested |
||
| Receivables |
283 |
Timing of reimbursement of sales taxes |
||
| Prepaid expenses |
1,835 |
Renewal of insurance contract and other prepaid expenses (advances to US vendors) |
||
| Assets held for sale |
(4) |
Foreign exchange |
||
| Right of use asset |
(43) |
Adjustment to the net present value of lease contract for Sherbrooke facility |
||
| Intangibles |
65,208 |
Related to acquisition of Grace (IPR&D) |
||
| Trade and other payables |
2,940 |
Increase in accrued expense related to the merger and timing of payments net of accruals |
||
| Derivative warrant liabilities |
(4,103) |
Change in fair value of derivative warrants |
||
| Lease liability |
(43) |
Payment of lease liability |
See the statement of changes in equity in our financial statements for details of changes to the equity accounts during the three-months periods ended September 30, 2021, and 2020.
Treasury Operations
Our treasury policy is to invest cash that is not required immediately into instruments with an investment strategy based on capital preservation. Cash equivalents and marketable securities are primarily made in guaranteed investment certificates, term deposits and high-interest savings accounts, which are issued and held with Canadian chartered banks, highly rated promissory notes issued by government bodies and commercial paper. We hold cash denominated in both U.S. and CAD dollars. Funds received in U.S. dollars from equity financings are invested as per our treasury policy in U.S. dollar investments and converted to CAD dollars as appropriate to fulfill operational requirements and funding.
Acquisition of Grace
On August 27, 2021, we completed our acquisition via merger of all outstanding equity interests in Grace Therapeutics Inc, a Delaware-incorporated, New Jersey-based rare and orphan disease specialty pharmaceutical company.
In connection with the share-for-share noncash transaction, Grace was merged with a new wholly owned subsidiary of Acasti and became a wholly owned subsidiary of Acasti. Grace was subsequently renamed Acasti Pharma US Inc. As a result of the merger, we acquired Grace’s entire therapeutic pipeline consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of various granted and pending patents in various jurisdictions worldwide. Under the terms of the merger, each issued and outstanding share of Grace common stock was automatically converted into the right to receive Acasti common shares equal to the equity exchange ratio set forth in the merger agreement.
| Total common shares issued |
18,241,233 | |||
| Acasti share price (closing share price on August 27, 2021) |
$ | 3.3344 | ||
| Fair value of common shares issued |
$ | 60,824 |
Our acquisition of Grace has been accounted for as a business combination using the acquisition method of accounting. This acquisition method requires, among other things, that assets acquired, and liabilities assumed in a business combination be recognized at their fair values as of the acquisition date. The valuation of assets acquired, and liabilities assumed has not yet been finalized as of September 30, 2021. As a result, we recorded preliminary estimates for the fair value of assets acquired and liabilities assumed as of the acquisition date. Finalization of the valuation during the measurement period could result in a change in the amounts recorded for the acquisition date fair value of intangible assets, goodwill, property and equipment, and income taxes among other items. The completion of the valuation will occur no later than one year from the acquisition date.
The following tables summarizes the preliminary fair value of assets acquired and liabilities assumed as of the acquisition date:
| Assets acquired and liabilities assumed |
||||
| Cash and equivalents |
$ | 90 | ||
| Prepaid expenses and other current assets |
$ | 74 | ||
| Intangible assets – In-process research and development |
$ | 65,208 | ||
| Accounts payable and accrued expenses |
$ | (4,548 | ) | |
| Total assets acquired and liabilities assumed |
$ | 60,824 |
Intangible assets of $65,208 relate primarily to the value of in-process research and development (“IPR&D”) related to Grace’s therapeutic pipeline, consisting of three unique clinical stage programs/assets and several preclinical assets supported by intellectual property.
Acquired In-Process Research and Development
In a business combination, the fair value of IPR&D acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as a definite-lived intangible asset or discontinued. If discontinued, the intangible asset will be written off. R&D costs incurred after the acquisition are expensed as incurred.
The estimated fair values of identifiable intangible assets were determined using the "income approach", which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. Some of the assumptions inherent in the development of these asset valuations include the estimated net cash flows for each year for the asset (including net revenues, cost of products sold, R&D costs, selling and marketing costs), the appropriate discount rate necessary to measure the risk inherent in each future cash flow stream, the life cycle of each asset, the potential regulatory and commercial success risk, competitive trends impacting the asset and each cash flow stream, as well as other factors.
Acquisition-related expenses, which were comprised primarily of regulatory, financial advisory and legal fees, totaled $2.5 million and $3.2 million, respectively, for the three and six-month periods ended September 30, 2021, and were included in general and administrative expenses in the condensed consolidated interim statements of earnings. The net loss attributed to Grace in the consolidated interim statement of income (loss) for the three- and six-months period ended September 30, 2021 since the date of acquisition is immaterial.
Pro Forma Financial Information
The following table presents the unaudited pro forma combined results of operations of Acasti and Grace for the six months ended September 30, 2021, as if the acquisition of Grace had occurred on April 1, 2020:
| Six months ended September 30, 2021 $ |
||||
| Net loss |
(5,892 | ) | ||
The unaudited pro forma condensed combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of Acasti and Grace. The unaudited pro forma financial information is not necessarily indicative of what the consolidated results of operations would have been had the acquisition been completed on April 1, 2020. In addition, the unaudited pro forma financial information is not a projection of future results of operations of the combined company, nor does it reflect the expected realization of any synergies or cost savings associated with the acquisition.
Assets Held for Sale
We determined to actively market for sale Other assets and Equipment and have met the criteria for classification of assets held for sale:
| September 30, 2021 |
March 31, 2021 |
|||||||
| $ | $ | |||||||
| Other assets |
384 | 387 | ||||||
| Equipment |
380 | 381 | ||||||
| 764 | 768 | |||||||
Other assets
Other assets represent krill oil (“RKO”) held by us that was expected to be used in commercial inventory scale up related to the development and commercialization of the CaPre drug candidate. Given that the development of CaPre will no longer be pursued by us, we expect to sell this reserve. The other asset is being recorded at the fair value less cost to sell. Management’s estimate of the fair value of the RKO less cost to sell was based primarily on estimated market prices at the end of our fiscal year ended March 31, 2021, obtained from an appraiser specializing in the krill oil market. Market prices have not changed materially since the end of our fiscal year ended March 31, 2021. These projections are based on Level 3 inputs of the fair value hierarchy and reflect management’s best estimate of market participants’ pricing of the assets as well as the general condition of the asset.
Equipment
| September 30, 2021 |
Cost, net of impairment |
Accumulated |
Net book |
|||||||||
| $ | $ | $ | ||||||||||
| Furniture and office equipment |
17 | (5 | ) | 12 | ||||||||
| Computer equipment |
96 | (29 | ) | 67 | ||||||||
| Laboratory equipment |
584 | (436 | ) | 148 | ||||||||
| Production equipment |
1,179 | (1,026 | ) | 153 | ||||||||
| 1,876 | (1,496 | ) | 380 | |||||||||
Equipment is made up of laboratory, production, computer, and office equipment that was utilized in the development of CaPre. Similarly, to the intangible assets and Other assets, the announcement of the failed Phase 3 clinical trials for CaPre resulted in an impairment trigger for the laboratory and production equipment. The impairment loss is based on management’s estimate of the fair value of the equipment less cost to sell, which is based primarily on estimated market prices obtained from brokers specialized in selling used equipment. These projections are based on Level 3 inputs of the fair value hierarchy and reflect our best estimate of market participants’ pricing of the assets as well as the general condition of the assets.
Derivative Warrant Liabilities
A total of 1,369,937 warrants were issued as part of our May 2018 public offering in Canada and recognized as derivative warrant liabilities with a fair value at inception of $3,323. As of September 30, 2021, the derivative warrant liability for the remaining 824,218 warrants totaled $615, which represents the fair value of these warrants as at September 30, 2021. The weighted average fair value of the warrants issued in the May 2018 public offering in Canada was determined to be CAD $3.10 per warrant at inception and approximately CAD $0.95 (US$0.75) per warrant as at September 30, 2021.
On December 27, 2017, 1,225,366 warrants were issued as part of our U.S. public offering and recognized as derivative warrant liabilities with a fair value at inception of $4,548. The December 2017 warrants are derivative warrant liabilities for accounting purposes due to the currency of the exercise price (US$) being different from our Canadian dollar functional currency. As of September 30, 2021, the derivative warrant liability for the remaining 884,120 warrants totaled $501, which represents the fair value of these warrants as at September 30, 2021. The weighted average fair value of the 2017 warrants issued was determined to be CAD $4.77 per warrant at inception and approximately CAD $0.72 (US$0.57) per warrant as at September 30, 2021.
The variance in the fair value of both existing derivative warrant liabilities as at September 30, 2021, is mostly due to the fluctuations in our share price and the dilution factor.
Contractual Obligations and Commitments
A summary of the contractual obligations at September 30, 2021, is as follows:
| Contractual Obligations |
Total |
Less than |
1 to 3 years |
More than |
||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Trade and other payables |
4,433 | 4,433 | - | - | ||||||||||||
| Operating lease obligations |
43 | 43 | - | - | ||||||||||||
| RKO supply agreement |
2,800 | 2,800 | - | - | ||||||||||||
| Total |
7,276 | 7,276 | - | - | ||||||||||||
Lease
On March 5, 2020, we renewed the lease agreement for our research and development and quality control laboratory facility located in Sherbrooke, Québec, resulting in an obligation of $160 over 24 months of the lease term. As at September 30, 2021, the remaining balance of the obligation amounted to $43.
RKO supply agreement
On October 25, 2019, we signed a supply agreement with Aker Biomarine Antartic. AS (“Aker”), to purchase raw krill oil product for a committed volume of commercial starting material for CaPre for a total value of $3.1 million (take or pay). The delivery of the products must be completed by October 31, 2021. As at September 30, 2021, the remaining balance of the commitment with Aker amounts to $2.8 million. There are no termination provisions within the supply agreement. As of November 10, 2021, no krill oil product has been delivered under the supply agreement, nor has any payment of the remaining balance of the commitment been made by Acasti. Management is currently assessing whether we can recover value from the raw krill oil product and given the uncertainty of recoverability, the Corporation expects it may incur a loss on this contract in the near term, absent an amended arrangement with the counterparty.
Contingencies
We evaluate contingencies on an ongoing basis and establish loss provisions for matters in which losses are probable and the amount of the loss can be reasonably estimated.
Off-Balance Sheet Arrangements
As of the date of this quarterly report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors, except for the RKO supply agreement.
Use of Estimates and Measurement of Uncertainty
The preparation of the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of derivative warrant liabilities, stock-based compensation, assets held for sale, acquisition of Grace valuation of intangibles and the take or pay contract. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date and determining which research and development expenses qualify for research and development tax credits and in what amounts. We recognize the tax credits once we have reasonable assurance that they will be realized. Recorded tax credits are subject to review and approval by tax authorities and, therefore, could be different from the amounts recorded. Estimates and assumptions are also utilized in the assessment of impairment of deferred financing costs, equipment, and intangibles.
Critical Accounting Policies
Valuation of Intangibles
In a business combination, the fair value of IPR&D acquired is capitalized and accounted for as indefinite-lived intangible assets, and not amortized until the underlying project receives regulatory approval, at which point the intangible assets will be accounted for as definite-lived intangible assets or discontinued. If discontinued, the intangible assets will be written off. R&D costs incurred after the acquisition are expensed as incurred.
The estimated fair values of identifiable intangible assets were determined using the "income approach", which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. Some of the assumptions inherent in the development of these asset valuations include the estimated net cash flows for each year for the asset (including net revenues, cost of products sold, R&D costs, and selling and marketing costs), the appropriate discount rate necessary to measure the risk inherent in each future cash flow stream, the life cycle of each asset, the potential regulatory and commercial success risk, competitive trends impacting the asset and each cash flow stream, as well as other factors.
Indefinite-lived assets are not amortized but are subject to an impairment review annually and more frequently when indicators of impairment exist. An impairment of indefinite-lived intangible assets would occur if the fair value of the intangible asset is less than the carrying value.
We test indefinite-lived intangible assets for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than its carrying amount, a quantitative impairment test is performed. For our quantitative impairment tests, we use an estimated future cash flow approach that requires significant judgment with respect to future sales volume, sales price and expense growth rates, changes in working capital use, the selection of an appropriate discount rate, asset groupings and other assumptions and estimates. The use of alternative estimates and assumptions could increase or decrease the estimated fair value of the assets and potentially result in different impacts to our results of operations. Actual results may differ from our estimates.
We review the recoverability of our definite-lived assets whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. The carrying amount is first compared with the undiscounted cash flows. If the carrying amount is higher than the sum of undiscounted cash flows, then we determine the fair value of the underlying asset group. Any impairment loss to be recognized is measured as the difference by which the carrying amount of the asset group exceeds the estimated fair value of the asset group.
Measurement of Assets Held for Sale
Assets that are classified as held for sale are measured at the lower of their carrying amount or fair value less expected selling costs (“estimated selling price”) with a loss recognized to the extent that the carrying amount exceeds the estimated selling price. The classification is applicable at the date upon which the sale of assets is probable, and the assets are available for immediate sale in their present condition. Assets, once classified as held for sale, are not subject to depreciation or amortization and both the assets and any liabilities directly associated with the assets held for sale are classified as current in our Consolidated Balance Sheets. Subsequent changes to the estimated selling price of assets held for sale are recorded as gains or losses to the Consolidated Statements of Income wherein the recognition of subsequent gains is limited to the cumulative loss previously recognized.
Financial Instruments
Credit Risk
Credit risk is the risk of a loss if a customer or counterparty to a financial asset fails to meet its contractual obligations. We have credit risk relating to cash, cash equivalents and marketable securities, which we manage by dealing only with highly rated financial institutions. The carrying amount of financial assets, as disclosed in the statements of financial position, represents our credit exposure at the reporting date.
Currency Risk
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of our business transactions denominated in currencies other than the Canadian dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in our operating results.
A portion of our expenses, mainly related to research contracts and purchase of production equipment, is incurred in U.S. dollars and in Euros, for which no financial hedging is required. There is a financial risk related to the fluctuation in the value of the U.S. dollar and the Euro in relation to the Canadian dollar. In order to minimize the financial risk related to the fluctuation in the value of the U.S. dollar in relation to the Canadian dollar, funds which were part of U.S. dollar financings continue to be invested as short-term investments in the U.S. dollar.
Furthermore, a portion of our cash and cash equivalents and marketable securities are denominated in U.S. dollars, further exposing us to fluctuations in the value of the U.S. dollar in relation to the Canadian dollar.
The following table provides an indication of our significant foreign exchange currency exposures as stated in Canadian dollars at the following dates:
| September 30, 2021 |
September 30, 2020 |
|||||||||||||||
| Denominated in |
US |
Euro |
US |
Euro |
||||||||||||
| Cash and cash equivalents |
42,127 | - | 5,913 | - | ||||||||||||
| Investments |
15,747 | - | - | - | ||||||||||||
| Trade and other payables |
(4,236 | ) | - | 3,443 | - | |||||||||||
| 53,638 | - | 9,356 | - | |||||||||||||
The following exchange rates are those applicable to the following periods and dates:
| September 30, 2021 |
September 30, 2020 |
|||||||||||||||
| Average |
Reporting |
Average |
Reporting |
|||||||||||||
| CAD$ per US$ |
1.2579 | 1.2680 | 1.3580 | 1.3319 | ||||||||||||
| CAD$ per Euro |
1.4852 | 1.4683 | 1.5418 | 1.5611 | ||||||||||||
Based on our foreign currency exposures noted above, varying the above foreign exchange rates to reflect a 5% strengthening of the U.S. dollar and Euro would have an increase (decrease) in net loss as follows, assuming that all other variables remain constant:
| September 30, 2021 |
September 30, 2020 |
|||||||
| $ | $ | |||||||
| Increase (decrease) in net loss |
3,401 | 623 | ||||||
An assumed 5% weakening of the foreign currencies would have an equal but opposite effect on the basis that all other variables remained constant.
Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market rates. Our exposure to interest rate risk as at September 30, 2021, and 2020 was as follows:
| Cash and cash equivalents |
Short-term fixed interest rate |
|
| Investments |
Short-term fixed interest rate |
Our capacity to reinvest the short-term amounts with equivalent return will be impacted by variations in short-term fixed interest rates available on the market. Management believes the risk we will realize a loss as a result of the decline in the fair value of our short-term investments is limited because these investments have short-term maturities and are held to maturity.
Liquidity Risk
Liquidity risk is the risk that we will not be able to meet our financial obligations as they fall due. We manage liquidity risk through the management of our capital structure and financial leverage. We also manage liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves our operating budgets and reviews material transactions outside the normal course of business.
Our contractual obligations related to financial instruments and other obligations and liquidity resources are presented in the liquidity and capital resources of this MD&A and note 1, nature of operations in the Financial Statements.
Future Accounting Changes
The following new standards and amendments to standards and interpretations are not yet effective for the period ended September 30, 2021, and have not been applied in preparing our consolidated financial statements.
In June 2016, the Financial Accounting Standards Board issued ASU 2016-13-Financial Instruments-Credit Losses (Topic 326), which amends guidance on reporting credit losses for assets held at amortized cost basis and available for sale debt securities. For assets held at amortized cost, the new guidance eliminates the probable initial recognition threshold in current U.S. GAAP and instead requires an entity to reflect its current estimate of all expected credit losses. The allowance for credit losses is a valuation account that is deducted from the amortized cost basis of the financial assets to present the net amount expected to be collected. ASU 2016-13 will affect loans, debt securities, trade receivables, net investments in leases, off balance sheet credit exposures, and any other financial assets not excluded from the scope that have the contractual right to receive cash. ASU 2016-13 is effective for annual periods, and interim periods within those annual periods, beginning after December 15, 2022. Management has not yet evaluated the impact of this ASU on our consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Information relating to quantitative and qualitative disclosures about market risks is detailed in “Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operation.”
Item 4. Controls and Procedures
Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report, our management, with the participation of our CEO and CFO, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15 (e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of September 30, 2021, our existing disclosure controls and procedures were effective. It should be noted that while our CEO and CFO believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, but not absolute, assurance that the objectives of the control system are met.
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the quarter ended September 30, 2021, that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting. The merger resulted in Grace being included in our current control environment over financial reporting as at the date of the completion of the business combination.
Item 1. Legal Proceedings
In the ordinary course of business, we are at times subject to various legal proceedings and disputes, including the proceedings specifically discussed below. We assess our liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that we will incur a loss and the amount of the loss can be reasonably estimated, we record a liability in our consolidated financial statements. These legal reserves may be increased or decreased to reflect any relevant developments on a quarterly basis. Where a loss is not probable or the amount of loss is not estimable, we do not accrue legal reserves. While the outcome of legal proceedings is inherently uncertain, based on information currently available and available insurance coverage, our management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on our financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to our financial position, results of operations, or cash flows.
Litigation Related to the Merger
In connection with the Grace merger, four stockholder lawsuits have been filed:
| (i) |
in the United States District Court for the Southern District of New York, captioned Bisel v. Acasti Pharma Inc. et al., Case No. 1:21-cv-06051 (the “Bisel Complaint”); |
|
| (ii) |
in the United States District Court for the District of Delaware, captioned Dawson v. Acasti Pharma Inc. et al., Case No. 1:21-cv-01039 (the “Dawson Complaint”); |
|
| (iii) |
in the United States District Court for the Eastern District of New York, captioned Weir v. Acasti Pharma Inc. et al., Case No. 1:21-cv-04151 (the “Weir Complaint”); and |
|
| (iv) |
in the United States District Court for the Southern District of New York, captioned Castaldo v. Acasti Pharma Inc. et al., Case No. 1:21-cv-06567 (the “Castaldo Complaint”) (together with the Bisel Complaint, the Dawson Complaint and the Weir Complaint, the “Complaints”)); |
The Complaints generally allege that our public disclosures pertaining to the Grace merger omit material facts in purported violation of Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder, and further that members of our Board of Directors are liable for those purported omissions under Section 20(a) of the Exchange Act. The relief sought in the Complaints includes, among other things, to enjoin the consummation of the merger pending disclosure of sufficient information, to award damages purportedly caused by the alleged omissions, and to award plaintiffs’ attorneys’ fees and other costs.
The Dawson and Weir Complaints have been voluntarily dismissed without prejudice. The Bisel and Castaldo Complaints have been consolidated. The Plaintiffs amended their Complaint in a consolidated action on October 1, 2021, to assert their claims on a class wide basis.
It is possible that additional lawsuits asserting similar claims could be filed. We strongly believe the allegations in the Complaints are frivolous and without merit, and plan to vigorously defend against them.
Any investment in our common shares involves a high degree of risk. The following risk factors and other information included in this quarterly report should be carefully considered. If any of these risks occur, our business, financial condition, prospects, results of operations or cash flow could be materially and adversely affected, and you could lose all or a part of the value of your investment. Additional risks or uncertainties not currently known to us, or that we deem immaterial, may also negatively affect our business operations.
Moreover, our recently completed acquisition of Grace is a significant business combination that substantially changed the profile of our company. As a result of the merger, below is a series of risk factors related to the ongoing business operations of the combined company that amends and restates in full the risk factors discussed in Part I, Item 1A. Risk Factors in our Form 10-K for the fiscal year ended March 31, 2021.
General Risks Related to the Company
We may not achieve our publicly announced milestones on time, or at all.
From time to time, we may publicly announce the timing of certain events that we expect to occur, such as the anticipated timing of results from our clinical trials and the timing of an upcoming NDA filing. These statements are forward-looking and are based on the best estimate of management at the time relating to the occurrence of the events. However, the actual timing of these events may differ from what has been publicly disclosed. The timing of events such as completion of a clinical trial, discovery of a new product candidate, filing of an application to obtain regulatory approval, beginning of commercialization of products, completion of a strategic partnership, or announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier or a distribution partner or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business, financial condition or operating results and the trading price of our common shares.
A failure to successfully integrate the businesses of Acasti and Acasti Pharma U.S. (formerly Grace) in the expected timeframe would adversely affect our future results.
The ongoing integration of the businesses of Acasti and Grace will be a time consuming and expensive process. Proper planning and effective and timely implementation will be critical to avoid any significant disruption to our operations. It is possible that the integration process could result in the loss of key employees, the disruption of our ongoing business or the identification of inconsistencies in standards, controls, procedures and policies that adversely affect our ability to maintain relationships with key opinion leaders, suppliers, manufacturers, creditors, lessors, clinical trial investigators or managers and other business partners or to otherwise not achieve the anticipated benefits of the merger. Delays encountered in the integration process could have a material adverse effect on our future revenue prospects, expenses, operating results and financial condition, including the value of our common shares.
Specifically, risks in integrating Acasti’s and Grace’s operations include, among other factors, our ability to effectively:
| • |
coordinate standards, compliance programs, controls, procedures and policies, business culture and compensation structures; |
|
| • |
integrate and harmonize financial reporting and information technology systems |
|
| • |
coordinate research and drug candidate development efforts; |
|
| • |
compete against companies pursing the same market opportunities expected to be available to the company following the merger; |
|
| • |
manage and eliminate inefficiencies associated with integrating the operations of the company; |
|
| • |
identify and eliminate redundant or underperforming personnel, operations, and assets; |
|
| • |
manage the diversion of management’s attention from business matters to integration issues; |
|
| • |
control additional costs and expenses in connection with, and as a result of the merger; |
|
| • |
conduct successful clinical development programs for our strategic product candidates and products and achieve regulatory approval for product candidates in major geographic areas; |
|
| • |
define and develop successful commercial strategies; |
|
| • |
commercialize products at commercially attractive margins and generate revenues in line with our expectations; and |
|
| • |
raise capital through equity or debt financing on attractive terms to support the development and commercialization of product candidates. |
In addition, the integration process may result in additional and unforeseen expenses, and the anticipated benefits of the integration plan may not be realized. If we are not able to adequately address these challenges, we may be unable to successfully integrate the operations of the business of Grace, or to realize the anticipated benefits. Our anticipated benefits from the merger assume a successful integration and are based on projections, which are inherently uncertain. Even if integration is successful, the ultimate benefits from the merger may not be as we expected.
Our future results will suffer if we do not effectively manage our expanded operations.
As a result of the merger, we have become a larger company than either of Acasti or Grace prior to the merger, and our business has become more complex. There can be no assurance that we will effectively manage the increased complexity without experiencing operating inefficiencies or control deficiencies. Significant management time and effort is required to effectively manage the increased complexity of the larger organization and if we fail to successfully do so it could have a material adverse effect on our business, financial condition, results of operations and growth prospects. In addition, as a result of the merger, our financial statements and results of operations in prior years may not provide meaningful guidance to form an assessment of our prospects or the potential success of our future business operations.
We have incurred and expect to continue to incur substantial expenses related to the merger and expect to continue to incur substantial expenses related to the integration of the companies.
There are a large number of ongoing post-merger processes, policies, procedures, operations, technologies and systems that must be integrated, including purchasing, accounting and finance, marketing, billing, payroll, research and development, human resources and benefits. In addition, the ongoing operation of locations in Laval and Sherbrooke, Québec and North Brunswick, New Jersey could result in inefficiencies, creating additional expense for us. While we have assumed that a certain level of expenses will be incurred, there are many factors beyond our control that could affect the total amount or timing of integration expenses. Moreover, many of the expenses that will be incurred are, by their nature, difficult to estimate accurately. These expenses could result in us taking significant charges against consolidated earnings, and the amount and timing of such charges are uncertain at present.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our suppliers, third party manufacturers and other contractors and consultants could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical pandemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to manufacture our products. Our ability to obtain supplies of candidate products could be disrupted if the operations of our manufacturers and suppliers are affected by a man-made or natural disaster or other business interruption.
We may be subject to foreign exchange rate fluctuations.
Our reporting currency is the U.S. dollar. However, many of our expenses currently are and/or are expected to be, denominated in foreign currencies, including Canadian dollars. As we previously completed financings in both Canadian and U.S. dollars, both currencies are maintained and used to make required payments in the applicable currency. Though we plan to implement measures designed to reduce our foreign exchange rate exposure, the U.S. dollar/Canadian dollar and U.S. dollar /European euro exchange rates have fluctuated significantly in the recent past and may continue to do so, which could have a material adverse effect on our business, financial position and results of operations.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our common shares could be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our common shares or publishes inaccurate or unfavorable research about our business, our share price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our shares could decrease, which could cause our share price and trading volume to decline.
Lawsuits have been filed, and other lawsuits may be filed, against us and members of our board of directors challenging the Grace merger, and an adverse ruling in any such lawsuit may result in an award of damages against us.
In connection with the Grace merger, four shareholder lawsuits have been filed. The lawsuits generally allege that our public disclosures pertaining to the Grace merger omit material facts in purported violation of Section 14(a) of the Exchange Act and Rule 14a-9 promulgated thereunder, and that members of our board of directors are liable for those purported omissions under Section 20(a) of the Exchange Act. The relief sought in the lawsuits includes, among other things, to enjoin the consummation of the merger, to award damages purportedly caused by the alleged omissions, and to award plaintiffs’ attorneys’ fees and other costs. It is possible that additional lawsuits asserting similar claims could be filed. We strongly believe the allegations in the lawsuits are frivolous and without merit, and plan to vigorously defend against them. The results of complex legal proceedings are difficult to predict. Moreover, the pending litigation is, and any future additional litigation could be, time consuming and expensive and could divert management’s attention away from its regular business.
Risk Factors Relating to our Business
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our executive team. Any of our executive officers could leave our employment at any time, as all of our employees are “at will” employees. Recruiting and retaining other qualified employees for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives and other personnel in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical companies for individuals with similar skill sets. In addition, failure to succeed in clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit key executives or the loss of the services of any executive or key employee might impede the progress of our development and commercialization objectives.
We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations and our ability to compete.
As our company matures, we expect to expand our employee base to increase our managerial, scientific, and engineering, operational, sales, marketing, financial and other resources and to hire more consultants and contractors. Future growth would impose significant additional responsibilities on our management, including the need to identify, recruit, maintain, motivate, and integrate additional employees, consultants and contractors. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Future growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our existing or future product candidates. Our future financial performance and our ability to sell and commercialize our product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage any future growth.
We face potential product liability, and if claims are brought against us, we may incur substantial liability.
The use of our product candidates in clinical trials (if any), and the sale of any drug candidates for which we obtain marketing approval, exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • |
impairment of our business reputation; |
| • |
withdrawal of clinical study participants; |
| • |
costs due to related litigation; |
| • |
distraction of management’s attention from our primary business; |
| • |
substantial monetary awards to patients or other claimants; and |
| • |
the inability to commercialize our product candidates. |
Our current product liability insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. A successful product liability claim or series of claims brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could adversely affect our results of operations and business.
We rely significantly on information technology and any failure, inadequacy, interruption, or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our internal computer systems, and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations and could result in a material disruption of our drug product development and clinical activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of drug product development or clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our development programs, and the development of our product candidates could be delayed.
Risks Related to Development, Testing and Commercialization of Our Products
Even if our drug candidates receive regulatory approval in the United States, we may never obtain regulatory approval or successfully commercialize our products outside of the United States.
Our business plan is highly dependent upon our ability to obtain regulatory approval market and commercialize our lead products, GTX-104, GTX-102 and GTX-101 in the United States. The failure to do so would have a material adverse effect on our ability to execute on our business plan and generate revenue. In addition, even if we obtain U.S. regulatory approvals to commercialize GTX-104, GTX-102 and GTX-101, we may not be able to do so in other international jurisdictions.
We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable to our drug candidates, could hinder or prevent our drug candidates’ commercial success.
Our ability to commercialize our product candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other third-party payors establish appropriate coverage and reimbursement levels for our product candidates and related treatments. As a threshold for coverage and reimbursement, third-party payors generally require that drug products have been approved for marketing by the FDA. Third-party payors are increasingly imposing additional requirements and restrictions on coverage and limiting reimbursement levels for medical products. These restrictions and limitations influence the purchase of healthcare services and products. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could significantly reduce our revenues from the sale of any approved product. We cannot provide any assurances that we will be able to obtain third-party coverage or reimbursement for our product candidates in whole or in part.
In the United States, there have been a number of legislative and regulatory changes to the healthcare system in ways that could affect our future revenues and profitability and the future revenues and profitability of our potential customers. Under the prescription drug benefit, Medicare beneficiaries can obtain prescription drug coverage from private sector plans that are permitted to limit the number of prescription drugs that are covered in each therapeutic category and class on their formularies. If our products are not widely included on the formularies of these plans, our ability to market our products to the Medicare population could be harmed.
There also have been, and likely will continue to be, legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare costs to contain or reduce costs of healthcare may adversely affect one or more of the following:
| • | our ability to set a price that we desire for our products; | |
| • | our ability to generate revenues and achieve profitability; | |
| • |
the future revenues of our potential customers, suppliers and collaborators; and |
|
| • |
the availability of capital. |
Any of these scenarios could harm our ability to market our products and generate revenues. It is also possible that other proposals having a similar effect will be adopted.
Our commercial success depends upon attaining significant market acceptance of our drug products and drug candidates, if approved, among physicians, nurses, pharmacists, patients and the medical community.
Even if we obtain regulatory approval for our drug product candidates, our drug product candidates may not gain market acceptance among physicians, nurses, pharmacists, patients, the medical community or third-party payors, which is critical to commercial success. Market acceptance of our drug products and any drug product candidate for which we receive approval depends on a number of factors, including:
| • |
the timing of market introduction of the product candidate as well as competitive products; |
|
| • |
the clinical indications for which the drug product candidate is approved; |
|
| • |
the convenience and ease of administration to patients of the drug product candidate; |
| • |
the potential and perceived advantages of such drug product candidate over alternative treatments; |
|
| • |
the cost of treatment in relation to alternative treatments, including any similar generic treatments; |
|
| • |
the availability of coverage and adequate reimbursement and pricing by third party payors and government authorities; |
|
| • |
relative convenience and ease of administration; |
|
| • |
any negative publicity related to our or our competitors’ products that include the same active ingredient; |
|
| • |
the prevalence and severity of adverse side effects, including limitations or warnings contained in a product’s FDA-approved labeling; and |
|
| • |
the effectiveness of sales and marketing efforts. |
If our drug products or drug candidates, if approved, fail to achieve an adequate level of acceptance by physicians, nurses, pharmacists, patients, and the medical community, we will be unable to generate significant revenues, and we may not become or remain profitable.
Guidelines and recommendations published by government agencies can reduce the use of our drug candidates and negatively impact our ability to gain market acceptance and market share.
Government agencies promulgate regulations and guidelines applicable to certain drug classes which may include our products and product candidates that we are developing. Recommendations of government agencies may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Regulations or guidelines suggesting the reduced use of certain drug classes which may include our products and product candidates that we are developing or the use of competitive or alternative products as the standard of care to be followed by patients and healthcare providers could result in decreased use of our product candidates or negatively impact our ability to gain market acceptance and market share.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug candidates, we may be unable to generate any revenue.
Although we intend to establish a small, focused, specialty sales and marketing organization to promote GTX-104 and GTX-102, if approved for marketing in the United States, we currently have no such organization and the cost of establishing and maintaining such an organization may exceed the benefit of doing so. Given the size of its potential market, we anticipate that to commercialize GTX-101, we would seek to enter into a strategic partnership with a larger marketing partner, if GTX-101 is approved by the FDA for marketing, and we may not be successful in doing so. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate sufficient product revenue and may not become profitable. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
If we obtain approval to commercialize any approved drug products outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If any of our drug candidates are approved for commercialization, we may enter into agreements with third parties to market these drug products outside the United States. We expect that we will be subject to additional risks related to entering into international business relationships, including:
| • |
different regulatory requirements for drug approvals in foreign countries; |
|
| • |
reduced protection for intellectual property rights; |
|
| • |
unexpected changes in tariffs, trade barriers and regulatory requirements; |
|
| • |
economic weakness, including inflation, or political instability in particular foreign economies and markets; |
|
| • |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
|
| • |
foreign taxes, including withholding of payroll taxes; |
|
| • |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
|
| • |
workforce uncertainty in countries where labor unrest is more common than in the United States; |
|
| • |
ability to secure third party marketing and selling agreements outside of the United States; |
|
| • |
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
|
| • |
business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
If we are unable to differentiate our drug candidates from branded reference drugs or existing generic therapies for similar treatments, or if the FDA or other applicable regulatory authorities approve generic products that compete with any of our drug candidates, our ability to successfully commercialize our drug candidates would be adversely affected.
Although we believe that our drug candidates will be clinically differentiated from branded reference drugs and their generic counterparts, if any, it is possible that such differentiation will not impact our market position. If we are unable to achieve significant differentiation for our product candidates against other drugs, the opportunity for our product candidates to achieve premium pricing and be commercialized successfully would be adversely affected.
In addition to existing branded reference drugs and the related generic products, the FDA or other applicable regulatory authorities may approve generic products that compete directly with our drug candidates, if approved. Once an NDA, including a 505(b)(2) application, is approved, the product covered thereby becomes a “listed drug” which can, in turn, be cited by potential competitors in support of approval of an approval of an abbreviated new drug application (“ANDA”). The Federal Food, Drug, and Cosmetic Act, FDA regulations and other applicable regulations and policies provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an ANDA for generic substitutes. These manufacturers might only be required to conduct a relatively inexpensive study to show that their product has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as our product candidate and that the generic product is bioequivalent to ours, meaning it is absorbed in the body at the same rate and to the same extent as our product candidate. These generic equivalents, which must meet the same quality standards as branded pharmaceuticals, would be significantly less costly than ours to bring to market and companies that produce generic equivalents are generally able to offer their products at lower prices. After the introduction of a generic competitor, a significant percentage of the sales of any branded product is typically lost to the generic product. Accordingly, competition from generic equivalents of our drug candidates would materially adversely impact our ability to successfully commercialize our drug candidates.
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical industry is intensely competitive and subject to rapid and significant technological change. We expect to have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions.
Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations. If our competitors market products that are more effective, safer or less expensive than our drug products, if any, or that reach the market sooner than our drug products, if any, we may enter the market too late in the cycle and may not achieve commercial success. In addition, the biopharmaceutical industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change, we may be unable to compete effectively. Technological advances or products developed by our competitors may render our technologies or product candidates obsolete, less competitive or not economical.
We could incur substantial costs and disruption to our business and delays in the launch of our drug candidates if our competitors and/or collaborators bring legal actions against us, which could harm our business and operating results.
We cannot predict whether our competitors or potential competitors, may bring legal actions against us based on our research, development, and commercialization activities, as well as any drug candidates or drug products resulting from these activities, claiming, among other things, infringement of their intellectual property rights, breach of contract or other legal theories. If we are forced to defend any such lawsuits, whether they are with or without merit or are ultimately determined in our favor, we may face costly litigation and diversion of technical and management personnel. These lawsuits could hinder our ability to enter the market early with our product candidates and thereby hinder our ability to influence usage patterns when fewer, if any, of our potential competitors have entered such market, which could adversely impact our potential revenue from such product candidates. Some of our competitors have substantially greater resources than we do and could be able to sustain the cost of litigation to a greater extent and for longer periods of time than we could. Furthermore, an adverse outcome of a dispute may require us: to pay damages, potentially including treble damages and attorneys’ fees, if we are found to have willfully infringed a party’s patent or other intellectual property rights; to cease making, licensing or using products that are alleged to incorporate or make use of the intellectual property of others; to expend additional development resources to reformulate our products or prevent us from marketing a certain drug; and to enter into potentially unfavorable royalty or license agreements in order to obtain the rights to use necessary technologies. Royalty or licensing agreements, if required, may be unavailable on terms acceptable to us, or at all.
The COVID-19 pandemic, or a similar pandemic, epidemic, or outbreak of an infectious disease, may materially and adversely affect our business and our financial results and could cause a disruption to the development of our drug candidates.
Public health crises such as pandemics or similar outbreaks could adversely impact our business. The coronavirus pandemic is evolving, and has led to the implementation of various responses, including government-imposed quarantines, travel restrictions and other public health safety measures. While to date, the coronavirus pandemic has not had a material adverse effect on our business, any negative impact COVID-19 has to patient enrollment or treatment, or the execution of our drug candidates could cause costly delays to clinical trial activities, which could adversely affect our ability to obtain regulatory approval for and to commercialize our drug candidates, increase our operating expenses, and have a material adverse effect on our financial results.
Additionally, timely enrollment in planned clinical trials is dependent upon clinical trial sites which could be adversely affected by global health matters, such as pandemics. We plan to conduct clinical trials for our drug candidates in geographies which are currently being affected by the COVID-19 pandemic. Some factors from the COVID-19 pandemic that will delay or otherwise adversely affect enrollment in the clinical trials of our drug candidates, as well as our business generally, include:
| • |
the potential diversion of healthcare resources away from the conduct of clinical trials to focus on pandemic concerns, including the attention of physicians serving as our clinical trial investigators, hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our prospective clinical trials; |
|
| • |
limitations on travel that could interrupt key trial and business activities, such as clinical trial site initiations and monitoring, domestic and international travel by employees, contractors or patients to clinical trial sites, including any government-imposed travel restrictions or quarantines that will impact the ability or willingness of patients, employees or contractors to travel to our clinical trial sites or secure visas or entry permissions, a loss of face-to-face meetings and other interactions with potential partners, any of which could delay or adversely impact the conduct or progress of our prospective clinical trials; |
|
| • |
the potential negative affect on the operations of our third-party manufacturers; |
|
| • |
interruption in global shipping affecting the transport of clinical trial materials, such as patient samples, investigational drug product and conditioning drugs and other supplies used in our prospective clinical trials; |
|
| • |
disruptions caused by potential workplace, laboratory and office closures and an increased reliance on employees working from home, disruptions to or delays in ongoing laboratory experiments; |
|
| • |
operations, staffing shortages, travel limitations or mass transit disruptions, any of which could adversely impact our business operations or delay necessary interactions with local regulators, ethics committees and other important agencies and contractors; |
|
| • |
changes in local regulations as part of a response to the COVID-19 pandemic, which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue such clinical trials altogether; and |
|
| • | interruption or delays in the operations of the FDA or other regulatory authorities, which may impact review and approval timelines. |
These factors arising from COVID-19 could worsen in countries that are already afflicted with COVID-19 or could continue to spread to additional countries. Any of these factors, and other factors related to any such disruptions that are unforeseen, could have a material adverse effect on our business and our results of operation and financial condition. Further, uncertainty around these and related issues could lead to adverse effects on the economy of the United States and other economies, which could impact our ability to raise the necessary capital needed to develop and commercialize our programs and drug candidates.
We are subject to numerous complex regulatory requirements and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business.
The research, testing, development, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, marketing, distribution, possession and use of our product candidates, among other things, are subject to regulation by numerous governmental authorities in the United States and elsewhere. The FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, and implementing regulations. Non-compliance with any applicable regulatory requirements can result in refusal of the governmental authority to approve products for marketing, criminal prosecution and fines, warning letters, product recalls or seizure of products, total or partial suspension of production, prohibitions or limitations on the commercial sale of products or refusal to allow the entering into of federal and state supply contracts. FDA and comparable governmental authorities have the authority to withdraw product approvals that have been previously granted. In addition, the regulatory requirements relating to our products may change from time to time and it is impossible to predict what the impact of any such changes may be.
We are heavily dependent on the success of our lead drug candidates, GTX-104, GTX-102 and GTX-101.
Our business and future success are substantially dependent on our ability to successfully and timely develop, obtain regulatory approval for, and commercialize our lead product candidate, GTX-104. Any delay or setback in the development of GTX-104 could adversely affect our business. Our planned development, approval and commercialization of GTX-104 may fail to be completed in a timely manner or at all. Our other product candidates, GTX-102 and GTX-101, are at an earlier development stage and we will require additional time and resources to develop and seek regulatory approval for such drug candidates and, if we are successful, to proceed with commercialization. We cannot provide assurance that we will be able to obtain approval for any of our drug candidates from the FDA or any foreign regulatory authority or that we will obtain such approval in a timely manner.
If the FDA does not conclude that our drug candidates satisfy the requirements for the 505(b)(2) regulatory approval pathway, or if the requirements for approval of any of our drug candidates under Section 505(b)(2) are not as we expect, the approval pathway for our drug candidates will likely take significantly longer, cost significantly more and encounter significantly greater complications and risks than anticipated, and in any case may not be successful.
We intend to seek FDA approval through the 505(b)(2) regulatory pathway for GTX-104, GTX-101 and GTX-102. The Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, added Section 505(b)(2) to the Federal Food, Drug and Cosmetic Act (“FDCA”). Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies that were not conducted by or for the applicant.
If the FDA does not allow us to pursue the 505(b)(2) regulatory pathway for GTX-104, GTX-101 or GTX-102 as anticipated, we may need to conduct additional clinical trials, provide additional data and information and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for our drug candidates would likely substantially increase. Moreover, the inability to pursue the 505(b)(2) regulatory pathway could result in new competitive products reaching the market faster than our drug candidates, which could materially adversely impact our competitive position and prospects. Even if we are allowed to pursue the 505(b)(2) regulatory pathway for a product candidate, we cannot assure you that we will receive the requisite or timely approvals for commercialization of such drug candidate.
In addition, it is possible that our competitors may file citizens’ petitions with the FDA in an attempt to persuade the FDA that our drug candidates, or the clinical studies that support their approval, contain deficiencies. Such actions by our competitors could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2).
Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Failure can occur at any stage of clinical development.
Clinical testing, even when utilizing the 505(b)(2) pathway, is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process, even with active ingredients that have previously been approved by the FDA as safe and effective. The results of preclinical studies and early clinical trials of our drug candidates may not be predictive of the results of later stage clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials.
Our drug candidates are in various stages of development. Clinical trial failures may occur at any stage and may result from a multitude of factors both within and outside our control, including flaws in formulation, adverse safety or efficacy profile and flaws in trial design, among others. If the trials result in negative or inconclusive results, we or our collaborators may decide, or regulators may require us, to discontinue trials of the product candidates or conduct additional clinical trials or preclinical studies. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent regulatory approval. For these reasons, our future clinical trials may not be successful.
Delays in clinical trials are common and have many causes, and any delay could result in increased costs to us and could jeopardize or delay our ability to obtain regulatory approval and commence product sales. We may also find it difficult to enroll patients in our clinical trials, which could delay or prevent development of our drug candidates.
We may experience delays in clinical trials of our drug candidates. Our planned clinical trials may not begin on time, have an effective design, enroll a sufficient number of patients or be completed on schedule, if at all. Our clinical trials can be delayed for a variety of reasons, including:
| • | inability to raise or delays in raising funding necessary to initiate or continue a trial; | |
| • | delays in obtaining regulatory approval to commence a trial; | |
| • |
delays in reaching agreement with the FDA on final trial design; |
|
| • |
imposition of a clinical hold for safety reasons or following an inspection of our clinical trial operations or trial sites by the FDA or other regulatory authorities; |
|
| • |
delays in reaching agreement on acceptable terms with prospective contract manufacturing organizations (CMOs), or contract research organizations (“CROs”), and clinical trial sites, or failure by such CMOs to complete the manufacturing of clinical trial materials or CROs to follow and carry out the clinical study protocol at each site in accordance with the terms of our agreements with them; |
|
| • |
delays in obtaining required institutional review board, or IRB, approval at each site; |
|
| • |
difficulties or delays in having patients’ complete participation in a trial or return for post-treatment follow-up; |
|
| • |
clinical sites electing to terminate their participation in one of our clinical trials, which would likely have a detrimental effect on subject enrollment; |
|
| • | time required to add new clinical sites; or | |
| • | delays by our contract manufacturers to produce and deliver sufficient supply of clinical trial materials. |
If initiation or completion of our planned clinical trials is delayed for any of the above reasons or other reasons, our development costs may increase, our regulatory approval process could be delayed and our ability to commercialize and commence sales of our drug candidates could be materially harmed, which could have a material adverse effect on our business.
In addition, identifying and qualifying patients to participate in clinical trials of our drug candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our drug candidates as well as completion of required follow-up periods. We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics or to complete our clinical trials in a timely manner. Patient enrollment is and completion of the trials are affected by a variety of factors, including:
| • |
severity and prevalence of the disease under investigation; |
|
| • |
design of the trial protocol; |
|
| • |
size of the patient population; |
|
| • |
eligibility criteria for the trial in question; |
|
| • |
perceived risks and benefits of the product candidate under trial; |
|
| • |
proximity and availability of clinical trial sites for prospective patients; |
|
| • |
availability of competing therapies and clinical trials; |
|
| • |
efforts to facilitate timely enrollment in clinical trials; |
|
| • |
patient referral practices of physicians; and |
|
| • |
ability to monitor patients adequately during and after treatment. |
Our drug products or drug candidates may cause adverse effects or have other properties that could delay or prevent their regulatory approval or limit the scope of any approved label or market acceptance, or result in significant negative consequences following marketing approval, if any.
As with many pharmaceutical and biological products, treatment with our drug products or product candidates may produce undesirable side effects or adverse reactions or events. Although the nature of our drug products or drug candidates as containing active ingredients that have already been approved means that the side effects arising from the use of the active ingredient or class of drug in our products or product candidates is generally known, our drug products or drug candidates may still cause undesirable side effects, which may harm our business, financial condition and prospects significantly.
Further, if any of our drug products cause serious or unexpected side effects after receiving market approval, a number of potentially significant negative consequences could result, including:
| • |
regulatory authorities may withdraw their approval of the product or impose restrictions on its distribution; |
|
| • |
the FDA may require implementation of a Risk Evaluation and Mitigation Strategy (“REMS”); |
|
| • |
regulatory authorities may require the addition of labeling statements, such as warnings or contraindications; |
|
| • |
we may be required to change the way the product is administered or conduct additional clinical studies; |
|
| • |
we could be sued and held liable for harm caused to patients; or |
|
| • |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product or product candidate and could substantially increase the costs of commercializing our products and product candidates.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our drug candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. It is possible that none of our existing drug candidates or any drug candidates we may seek to develop will ever obtain regulatory approval in the United States or other jurisdictions.
Our drug candidates could fail to receive regulatory approval for many reasons, including the following:
| • |
the FDA or comparable foreign regulatory authorities may disagree that our changes to branded reference drugs meet the criteria for the 505(b)(2) regulatory pathway or foreign regulatory pathways; |
|
| • |
we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a drug candidate is safe and effective or comparable to its branded reference product for its proposed indication; |
|
| • |
the results of any clinical trials we conduct may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
|
| • |
we may be unable to demonstrate that a drug candidate’s clinical and other benefits outweigh its safety risks; |
|
| • |
the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
|
| • |
the approval policies or regulations of the FDA or comparable foreign regulatory authorities may change significantly in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process as well as the unpredictability of future clinical trial results may result in us failing to obtain regulatory approval to market our drug candidates, which would harm our business, results of operations and prospects significantly.
We have limited experience using the 505(b)(2) regulatory pathway to submit an NDA or any similar drug approval filing to the FDA, and we cannot be certain that any of our drug candidates will receive regulatory approval. If we do not receive regulatory approvals for our drug candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our drug candidates, our revenue will be dependent, to a significant extent, upon the size of the markets in the territories for which we gain regulatory approval. If the markets for patients or indications that we are targeting are not as significant as we estimate, we may not generate significant revenue from sales of such drug products, if approved.
An NDA submitted under Section 505(b)(2) subjects us to the risk that we may be subject to a patent infringement lawsuit that would delay or prevent the review or approval of our drug candidate. The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
Our drug candidates will be submitted to the FDA for approval under Section 505(b)(2) of the FDCA. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies that were not conducted by, or for, the applicant and on which the applicant has not obtained a right of reference. The 505(b)(2) application would enable us to reference published literature and/or the FDA’s previous findings of safety and effectiveness for the branded reference drug. For NDAs submitted under Section 505(b)(2) of the FDCA, the patent certification and related provisions of the Hatch-Waxman Act apply. In accordance with the Hatch-Waxman Act, such NDAs may be required to include certifications, known as paragraph IV certifications, that certify that any patents listed in the Patent and Exclusivity Information Addendum of the FDA’s publication, Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, with respect to any product referenced in the 505(b)(2) application, are invalid, unenforceable or will not be infringed by the manufacture, use or sale of the product that is the subject of the 505(b)(2) NDA.
Companies that produce branded reference drugs routinely bring litigation against 505(b)(2) applicants that seek regulatory approval to manufacture and market generic and reformulated forms of their branded products. These companies often allege patent infringement or other violations of intellectual property rights as the basis for filing suit against a 505(b)(2) applicant. Likewise, patent holders may bring patent infringement suits against companies that are currently marketing and selling their approved generic or reformulated products. When a drug, such as GTX-104, has orphan drug exclusivity, the FDA may not approve any other application to market the same drug for the same indication for a period of up to seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity. In the United States, pediatric exclusivity adds six months to any existing exclusivity period.
Our business is subject to extensive regulatory requirements and our drug candidates that obtain regulatory approval will be subject to ongoing and continued regulatory review, which may result in significant expense and limit our ability to commercialize such products.
Even after a drug product is approved, we will remain subject to ongoing FDA and other regulatory requirements governing the labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, import, export, record-keeping and reporting of safety and other post-market information. The holder of an approved NDA is obligated to monitor and report adverse events, and any failure of a drug product to meet the specifications in the NDA. The holder of an approved NDA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA laws and regulations and are subject to FDA review, in addition to other potentially applicable federal and state laws. In addition, the FDA may impose significant restrictions on the approved indicated uses for which the product may be marketed or on the conditions of approval. For example, a product’s approval may contain requirements for potentially costly post-approval studies and surveillance to monitor the safety and efficacy of the product, or the imposition of a REMS program.
In addition, the FDA’s regulations, policies, or guidance may change and new or additional statutes or government regulations in the United States and other jurisdictions may be enacted that could prevent or delay regulatory approval of our product candidates or further restrict or regulate post-approval activities. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from pending or future legislation or administrative action, either in the United States or abroad. If we are not able to achieve and maintain regulatory compliance, we may not be permitted to market our products and/or product candidates, which would adversely affect our ability to generate revenue and achieve or maintain profitability.
Our employees, independent contractors, principal investigators, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, principal investigators, consultants, commercial partners, and vendors may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct that violates (1) the laws of the FDA and similar foreign regulatory bodies, including those laws requiring the reporting of true, complete, and accurate information to such regulatory bodies; (2) healthcare fraud and abuse laws of the United States and similar foreign fraudulent misconduct laws; and (3) laws requiring the reporting of financial information or data accurately. Specifically, the promotion, sales and marketing of health care items and services, as well as certain business arrangements in the healthcare industry are subject to extensive laws designed to prevent misconduct, including fraud, kickbacks, self-dealing and other abusive practices. These laws may restrict or prohibit a wide range of pricing, discounting, marketing, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials. It is not always possible to identify and deter employee and other third-party misconduct. The precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws. If any such actions are instituted against us, and we are not successful in defending ourselves, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Any relationships with healthcare professionals, principal investigators, consultants, customers (actual and potential) and third-party payors are and will continue to be subject, directly, or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, marketing expenditure tracking and disclosure, or sunshine laws, government price reporting and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations.
Our business operations and activities may be directly, or indirectly, subject to various federal, state, and local fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. These laws may impact, among other things, our current activities with principal investigators and research subjects, as well as proposed and future sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by the federal government, state governments and foreign jurisdictions in which we conduct our business. The laws that may affect our ability to operate include, but are not limited to:
| • |
the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs; |
|
| • |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other third-party payors that are false or fraudulent or knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government; |
|
| • |
the federal Health Insurance Portability and Accountability Act of 1996, or (“HIPAA,”), which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; | |
| • |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or (“HITECH,”), and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization; |
| • |
the federal Physician Payment Sunshine Act, created under Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, (collectively, “ACA,”), and its implementing regulations requires manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services, or HHS, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) (and beginning on January 1, 2021 this also includes Physician Assistants, Nurse Practitioners, Clinical Nurse Specialists, Certified Registered Nurse Anesthetists, and Certified Nurse Midwives (CNM) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, with data collection required beginning August 1, 2013 and reporting to the Centers for Medicare & Medicaid Services required by March 31, 2014 and by the 90th day of each subsequent calendar year; |
|
| • |
federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
|
| • |
federal government price reporting laws, changed by ACA to, among other things, increase the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program and offer such rebates to additional populations, that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our marketed drugs. Participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs and potentially limit our ability to offer certain marketplace discounts; |
|
| • | the Foreign Corrupt Practices Act, a United States law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and | |
| • |
state law equivalents of each of the above federal laws. |
In addition, any sales of our drug products or drug candidates once commercialized outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to, without limitation, civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
We are required to obtain regulatory approval for each of our drug candidates in each jurisdiction in which we intend to market such products, and the inability to obtain such approvals would limit our ability to realize their full market potential.
Risks Relating to Our Intellectual Property
If we are sued for infringing intellectual property rights of third parties, it will be costly and time consuming, and an unfavorable outcome in that litigation would have a material adverse effect on our business.
Our commercial success also depends upon our ability and the ability of our future collaborators to develop, manufacture, market and sell our product candidates and to use our proprietary technologies without infringing the proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing products. Because patent applications can take many years to issue, there may be currently pending applications, which may later result in issued patents that our product candidates or proprietary technologies may infringe. Similarly, there may be issued patents relevant to our product candidates of which we are not aware.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and biopharmaceutical industries generally. In particular, the generic drug industry is characterized by frequent litigation between generic drug companies and branded drug companies. If a third-party claims that we infringe its intellectual property rights, we may face a number of issues, including, but not limited to:
| • |
infringement and other intellectual property claim which, with or without merit, may be expensive and time-consuming to litigate and may divert our management’s attention from our core business; |
|
| • |
substantial damages for infringement, including, but not limited to, treble damages, punitive damages, loss of profits and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes on or violates the third party’s rights; |
|
| • |
if a license is available from the third party, we may have to pay substantial royalties, fees and/or grant cross licenses to our technology; and |
|
| • |
redesigning our product candidates or processes so they do not infringe, which may not be possible or may require substantial funds and time. |
We have not conducted an extensive search of patents issued to third parties, and no assurance can be given that third party patents containing claims covering our product candidates, technology or methods do not exist, have not been filed, or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas or fields, we believe there is a significant risk that third parties may allege they have patent rights encompassing our products, technology, or methods. Other product candidates that we may in-license or acquire could be subject to similar risks and uncertainties.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed alleged confidential information or trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, certain of our employees were formerly employed by other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Moreover, we engage the services of consultants to assist us in the development of our product candidates, many of whom were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees and consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. There can be no assurance, however, that we would not be sued. Any such litigation would be protracted, expensive, and potentially subject to an unfavorable outcome.
Our success depends in part upon our ability to protect our intellectual property for our branded products and drug candidates, such as GTX-104, GTX-102 and GTX-101.
Our commercial success with respect to our drug products and drug candidates, including GTX-104, GTX-102 and GTX-101, depends on obtaining and maintaining patent protection in both the United States and in other countries and trade secret protection for our product candidates, proprietary technologies and their uses. Our ability to protect our drug products from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents.
Due to evolving legal standards relating to patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to maintain, obtain and enforce patents is uncertain and involves complex legal and factual questions. The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value and the scope of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents.
The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| • |
we might not have been the first to file patent applications for these or similar inventions; |
|
| • |
we might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
|
| • |
others may independently develop similar or alternative technologies or duplicate any of our technologies; |
|
| • |
the patents of others may have an adverse effect on our business; |
|
| • |
it is possible that none of our or our licensors’ pending patent applications will result in issued patents; |
|
| • |
any patents we obtain, or our licensors’ issued patents may not encompass commercially viable products, may not provide us with any competitive advantages, or may be challenged by third parties for lack of novelty, obviousness, lack of demonstrated or predicted utility, or other technical reasons related to the drafting of the patent itself; |
|
| • |
any patents we obtain, or our in-licensed issued patents may not be valid or enforceable; or |
|
| • |
we may not develop additional proprietary technologies that are patentable. |
Proprietary trade secrets and unpatented know-how are also very important to our business. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with certain of our employees, consultants, and advisors, third parties may still obtain this information, or we may be unable to protect our rights. Enforcing a claim that a third party illegally obtained and is using our trade secrets or unpatented know-how is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secret information. Moreover, our competitors may independently develop equivalent knowledge, methods, and know-how, and we would not be able to prevent their use.
Our drug development strategy relies heavily upon the 505(b)(2) regulatory pathway, which requires us to certify that we do not infringe upon third-party patents covering approved drugs. Such certifications typically result in third-party claims of intellectual property infringement, the defense of which will be costly and time consuming, and an unfavorable outcome in any litigation may prevent or delay our development and commercialization efforts which would harm our business.
Litigation or other proceedings to enforce or defend intellectual property rights are often complex in nature, may be very expensive and time-consuming, may divert our management’s attention from other aspects of our business and may result in unfavorable outcomes that could adversely impact our ability to launch and market our product candidates, or to prevent third parties from competing with our products and product candidates.
In particular, our commercial success depends in large part on our avoiding infringement of the patents and proprietary rights of third parties for existing approved drug products. Because we utilize the 505(b)(2) regulatory pathway for the approval of our products and product candidates, we rely in whole or in part on studies conducted by third parties related to those approved drug products.
Because patent applications can take many years to issue, there may be currently pending or subsequently filed patent applications which may later result in issued patents that may be infringed by our products or product candidates. If any third-party patents were held by a court of competent jurisdiction to cover aspects of our product candidates, including the formulation, method of use, any method or process involved in the manufacture of any of our product candidates, any molecules or intermediates formed during such manufacturing process or any other attribute of the final product itself, the holders of any such patents may be able to block our ability to commercialize our product candidates unless we obtain a license under the applicable patents, or until such patents expire. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may request and/or obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates on a temporary or permanent basis. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products or manufacturing processes, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research, manufacture clinical trial supplies or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our product candidates, which could harm our business significantly. We cannot provide any assurances that third party patents do not exist which might be enforced against our products, resulting in either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties.
If we fail to comply with our obligations in the agreements under which we license rights to technology from third parties, or if the license agreements are terminated for other reasons, we could lose license rights that are important to our business.
We may be a party to a number of technology licenses that are important to our business and expect to enter into additional licenses in the future. Our existing license agreements impose, and we expect that future license agreements will impose, on us, various development, regulatory and/or commercial diligence obligations, payment of milestones and/or royalties and other obligations. Under these agreements, we must rely on our licensor to comply with their obligations under the primary license agreements under which such third party obtained rights in the applicable intellectual property, where we may have no relationship with the original licensor of such rights. If our licensors fail to comply with their obligations under these upstream license agreements, the original third-party licensor may have the right to terminate the original license, which may terminate our sublicense. If this were to occur, we would no longer have rights to the applicable intellectual property unless we are able to secure our own direct license with the owner of the relevant rights, which we may not be able to do at a reasonable cost or on reasonable terms, which may impact our ability to continue to develop and commercialize our product candidates and companion diagnostic incorporating the relevant intellectual property. If we fail to comply with our obligations under our license agreements, or we are subject to a bankruptcy or insolvency, the licensor may have the right to terminate the license. In the event that any of our important technology licenses were to be terminated by the licensor, we would likely cease further development of the related program or be required to spend significant time and resources to modify the program to not use the rights under the terminated license.
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. We may also be subject to claims that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We may be subject to ownership disputes in the future arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates and companion diagnostic. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | others may be able to make compounds that are similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; | |
| • |
we or our licensors or future collaborators might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
|
| • |
we or our licensors or future collaborators might not have been the first to file patent applications covering certain of our inventions; |
|
| • |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
|
| • |
it is possible that our pending patent applications will not lead to issued patents; |
|
| • |
issued patents that we own or have exclusively licensed may be held invalid or unenforceable as a result of legal challenges by our competitors; |
|
| • |
we may not develop additional proprietary technologies that are patentable; and |
|
| • |
the patents of others may have an adverse effect on our business. |
Should any of these events occur, they could significantly harm our business, results of operations and prospects.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect any of our other future drug candidates.
Numerous recent changes to the patent laws and proposed changes to the rules of the various patent offices around the world may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. These changes may lead to increasing uncertainty with regard to the scope and value of our issued patents and to our ability to obtain patents in the future.
Once granted, patents may remain open to opposition, re-examination, post-grant review, inter partes review, nullification derivation and opposition proceedings in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against the initial grant. In the course of any such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims attacked or may lose the allowed or granted claims altogether. Depending on decisions by authorities in various jurisdictions, the laws and regulations governing patents could change in unpredictable ways that may weaken our and our licensors’ ability to obtain new patents or to enforce existing patents we and our licensors or partners may obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Dependence on Third Parties
We do not have internal manufacturing capabilities, and if we fail to develop and maintain supply relationships with various third-party manufacturers, we may be unable to develop or commercialize our drug candidates.
Our ability to develop and commercialize our product candidates depends, in part, on our ability to outsource their manufacturing at a competitive cost, in accordance with regulatory requirements and in sufficient quantities for clinical testing and eventual commercialization. All of our manufacturing is outsourced to third parties, and we do not plan to build manufacturing capabilities.
Our contract manufacturers may encounter manufacturing failures that could delay the clinical development or regulatory approval of our drug candidates, or their commercial production, if approved.
Any performance failure on the part of any of our manufacturers could delay the clinical development or regulatory approval of our product candidates. Our manufacturers may encounter difficulties involving, among other things, production yields, regulatory compliance, quality control and quality assurance, as well as shortages of qualified personnel. Approval of our product candidates could be delayed, limited, or denied if the FDA does not approve and maintain the approval of our contract manufacturer’s processes or facilities. Moreover, our contract manufacturers may encounter difficulties that have a negative impact on our operations and business. Our manufacturers may encounter difficulties with the manufacturing processes required to manufacture commercial quantities of our product candidates or the quantities needed for our pre-clinical studies or clinical trials. Such difficulties could result in delays in our pre-clinical studies, clinical trials, and regulatory submissions, in the commercialization of our product candidates. Further, development of large-scale manufacturing processes may require additional validation studies, which the FDA must review and approve. If any of our manufacturers fail to deliver the required commercial quantities or quantities needed for our pre-clinical studies and clinical trials on a timely basis and upon terms that we find acceptable, we may be unable to meet demand for any of our product candidates that are approved and could lose potential revenue.
Certain changes in the manufacturing process or procedure, including a change in the location where the product candidate is manufactured or a change of a third-party manufacturer, generally require prior FDA, or foreign regulatory authority, review and/or approval of the manufacturing process and procedures in accordance with cGMP. We may need to conduct additional pre-clinical studies and clinical trials to support approval of such changes. This review may be costly and time-consuming and could delay or prevent the launch of a product candidate.
We rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our drug candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third party CROs to monitor and manage data for our preclinical and clinical programs. We rely on these parties for execution of our preclinical studies and clinical trials, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with FDA laws and regulations regarding current good clinical practice (“GCP”), which are also required by the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities in the form of International Conference on Harmonization, guidelines for all of our products in clinical development. Regulatory authorities enforce GCP through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or any of our CROs fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. While we have agreements governing activities of our CROs, we have limited influence over their actual performance. In addition, portions of the clinical trials for our product candidates are expected to be conducted outside of the United States, which will make it more difficult for us to monitor CROs and perform visits of our clinical trial sites and will force us to rely heavily on CROs to ensure the proper and timely conduct of our clinical trials and compliance with applicable regulations, including GCP. Failure to comply with applicable regulations in the conduct of the clinical trials for our product candidates may require us to repeat clinical trials, which would delay the regulatory approval process.
We rely on third parties to manufacture commercial and clinical supplies of our drug candidates, and we intend to rely on third parties to manufacture commercial supplies of any approved drug products. The commercialization of any of our drug products could be stopped, delayed, or made less profitable if those third parties fail to provide us with sufficient quantities of active pharmaceutical ingredients, excipients, or drug products, or fail to do so at acceptable quality levels or prices or fail to maintain or achieve satisfactory regulatory compliance.
We do not own any manufacturing facilities, and we do not currently, and do not expect in the future, to independently conduct any aspects of our product manufacturing and testing, or other activities related to the clinical development and commercialization of our drug candidates. We currently rely, and expect to continue to rely, on third parties with respect to these items, and control only certain aspects of their activities.
Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it could delay our product candidate development and commercialization activities. Our reliance on these third parties reduces our control over these activities but does not relieve us of our responsibility to ensure compliance with all required legal, regulatory, and scientific standards and any applicable trial protocols. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our studies in accordance with regulatory requirements or our stated study plans and protocols, we will not be able to complete, or may be delayed in completing, clinical trials required to support future regulatory submissions and approval of our drug candidates.
More generally, manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up and validating initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, shortages of qualified personnel, as well as compliance with strictly enforced federal, state, and foreign regulations. Additionally, our manufacturers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes or unstable political environments. If our manufacturers were to encounter any of these difficulties, or otherwise fail to comply with their contractual obligations, our ability to make product candidates available for clinical trials and development purposes or to commercialize any of our product candidates in the United States would be jeopardized. Any delay or interruption in our ability to meet commercial demand may result in the loss of potential revenues and could adversely affect our ability to gain market acceptance for approved products. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of clinical trials, increase the costs associated with maintaining clinical trial programs and, depending upon the period of delay, require us to commence new clinical trials at additional expense or terminate clinical trials completely. Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. Regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
The design, development, manufacture, supply, and distribution of our drug candidates is highly regulated and technically complex.
All entities involved in the preparation of therapeutics for clinical trials or commercial sale, including our existing contract manufacturers for our drug candidates, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP and equivalent foreign standards. These regulations govern manufacturing processes and procedures (including record-keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our drug candidates that may not be detectable in final product testing. The development, manufacture, supply, and distribution of our product candidates is highly regulated and technically complex. We, along with our third-party providers, must comply with all applicable regulatory requirements of the FDA and foreign authorities.
Regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business. If we or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new drug product or biological product or revocation of a pre-existing approval. As a result, our business, financial condition, and results of operations may be materially harmed.
We may not be successful in establishing development and commercialization collaborations which could adversely affect, and potentially prohibit, our ability to develop our drug candidates.
Because developing pharmaceutical products, conducting clinical trials, obtaining regulatory approval, establishing manufacturing capabilities and marketing approved products are expensive, we are exploring collaborations with third parties outside of the United States that have more resources and experience. In situations where we enter into a development and commercial collaboration arrangement for a drug candidate, we may also seek to establish additional collaborations for development and commercialization in territories outside of those addressed by the first collaboration arrangement for such drug candidate. There are a limited number of potential partners, and we expect to face competition in seeking appropriate partners. If we are unable to enter into any development and commercial collaborations and/or sales and marketing arrangements on acceptable terms, if at all, we may be unable to successfully develop and seek regulatory approval for our drug candidates and/or effectively market and sell future approved drug products, if any, in all of the territories outside of the United States where it may otherwise be valuable to do so.
We may not be successful in maintaining development and commercialization collaborations, and any partner may not devote sufficient resources to the development or commercialization of our drug candidates or may otherwise fail in development or commercialization efforts, which could adversely affect our ability to develop certain of our drug candidates and our financial condition and operating results.
Even if we are able to establish collaboration arrangements, any such collaboration may not ultimately be successful, which could have a negative impact on our business, results of operations, financial condition and prospects. If we partner with a third party for development and commercialization of a drug candidate, we can expect to relinquish some or all of the control over the future success of that drug candidate to the third party. It is possible that a partner may not devote sufficient resources to the development or commercialization of our product candidate or may otherwise fail in development or commercialization efforts, in which event the development and commercialization of such product candidate could be delayed or terminated, and our business could be substantially harmed. In addition, the terms of any collaboration or other arrangement that we establish may not prove to be favorable to us or may not be perceived as favorable, which may negatively impact the trading price of our common stock. In some cases, we may be responsible for continuing development of a product candidate or research program under a collaboration, and the payment we receive from our partner may be insufficient to cover the cost of this development. Moreover, collaborations and sales and marketing arrangements are complex and time consuming to negotiate, document and implement, and they may require substantial resources to maintain.
We are subject to a number of additional risks associated with our dependence on collaborations with third parties, the occurrence of which could cause our collaboration arrangements to fail. Conflicts may arise between us and our partners, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any such conflicts arise, a partner could act in its own self-interest, which may be adverse to our interests. Any such disagreement between us and a partner could result in one or more of the following, each of which could delay or prevent the development or commercialization of our drug candidates and harm our business:
| • |
reductions in the payment of royalties or other payments we believe are due pursuant to the applicable collaboration arrangement; |
|
| • |
actions taken by a partner inside or outside our collaboration which could negatively impact our rights or benefits under our collaboration; and |
|
| • |
unwillingness on the part of a partner to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities. |
Risks Relating to Our Common Shares
We may be treated as a passive foreign investment corporation for U.S. federal income tax purposes.
We have not yet determined whether we will be a PFIC for our taxable year that includes the merger or the likelihood that we will be a PFIC in future taxable years, but we believe that we may not be classified as a PFIC for the current taxable year or future taxable years. However, the determination PFIC status of a non-U.S. corporation is fundamentally factual in nature, depends on the application of complex U.S. federal income tax rules (which are subject to differing interpretations), generally cannot be determined until the close of the taxable year in question and is determined annually. Accordingly, there can be no assurance that we will not be a PFIC in our current taxable year or subsequent years. The PFIC rules are complex, and each U.S. shareholder should consult its tax advisor regarding the application of the PFIC rules to us as well as the potential impact of the PFIC rules on such holder if we were determined to be a PFIC.
The rules governing PFICs can have adverse tax effects on U.S. shareholders, which effects may be mitigated by making certain elections for U.S. federal income tax purposes, which elections may or may not be available. If we are a PFIC in any year, a U.S. shareholder in such year will be required to file an annual information return with the IRS-on-IRS Form 8621 regarding distributions received on such common shares, any gain realized on disposition of such common shares and any other information required by such form. Additionally, if we are classified as a PFIC in any taxable year with respect to which a U.S. shareholder owns common shares, we generally will continue to be treated as a PFIC with respect to such U.S. shareholder in all succeeding taxable years, regardless of whether we continue to meet the tests described above, unless the U.S. shareholder makes a “deemed sale election.”.
We may not be able to use our net operating loss carryforwards to offset future taxable income for Canadian or U.S. federal income tax purposes.
At September 30, 2021, Acasti Pharma U.S. had net operating loss carryforwards (“NOLs”) for U.S. federal income tax purposes of approximately $9.1 million, which begin to expire in 2038.
Acasti Pharma U.S. underwent an “ownership change” within the meaning of Section 382 of the Code as a result of the merger, and therefore Acasti Pharma U.S. may become subject to an annual limit on the amount of NOLs that may be used to offset future taxable income of Acasti Pharma U.S. for U.S. federal income tax purposes. Such annual limit is generally equal to the product of (i) the total value of the loss company’s (in this case, Acasti Pharma U.S.) outstanding equity immediately prior to an “ownership change” (subject to certain adjustments); and (ii) the applicable federal long-term tax-exempt interest rate for the month that includes the “ownership change”.
At September 30, 2021, we had NOLs for Canadian federal income tax purposes of approximately $108.3 million, which expire at various dates through 2041. The extent to which we can utilize any or all of our NOLs will depend on many factors, including the jurisdiction applicable to any of our future taxable revenue.
Our ability to use NOLs will also depend on the amount of taxable income generated in future periods. The NOLs may expire before we can generate sufficient taxable income to use the NOLs.
We do not expect to pay any cash dividends for the foreseeable future.
The continued operation and expansion of our business will require substantial funding. Accordingly, we do not anticipate that we will pay any cash dividends on our common shares for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
The price of our common shares may be volatile.
Market prices for securities of pharmaceutical companies can fluctuate significantly. Factors such as the announcement to the public or in various scientific or industry forums of technological innovations; new commercial products; patents or exclusive rights obtained by us or others; disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; the commencement, enrollment or announcement of results of clinical trials we conduct, or changes in the development status of our product candidates; results or delays of pre-clinical and clinical studies by us or others; any delay in our regulatory filings for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings; a change of regulations; additions or departures of key scientific or management personnel; overall performance of the equity markets; general political and economic conditions; publications; failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public; research reports or positive or negative recommendations or withdrawal of research coverage by securities analysts; actual or anticipated variations in quarterly operating results; announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; public concerns over the risks of pharmaceutical products and dietary supplements; unanticipated serious safety concerns related to the use of our products; our access to financial resources, future sales of securities by us or our shareholders; and many other factors, many of which are beyond our control, could have considerable effects on the price of our common shares. The price of our common shares has fluctuated significantly in the past and there can be no assurance that the market price of our common shares will not experience significant fluctuations in the future.
In addition, securities of pharmaceutical companies often experience extreme price and volume fluctuations that are unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may negatively affect the market price of our common shares, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against pharmaceutical companies following periods of volatility in the market price of their securities. This type of litigation, if instituted against us, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations, or require us to relinquish rights to our technologies or product candidates.
We will need to raise additional capital in the future in order to fully execute on our business plan. We may seek additional capital through a combination of public and private equity offerings, debt financings, and non-dilutive strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. We have in place an “at-the-market” sales agreement where we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000. The incurrence of indebtedness by us would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us.
The market price of our common shares could decline as a result of operating results falling below the expectations of investors or fluctuations in operating results each quarter.
Our net losses and expenses may fluctuate significantly and any failure to meet financial or clinical expectations may disappoint securities analysts or investors and result in a decline in the price of our common shares. Our net losses and expenses have fluctuated in the past and are likely to do so in the future. The market price of our common shares has fluctuated significantly in the past and may continue to do so. Some of the factors that could cause the market price for our common shares to fluctuate include the following:
| • |
results of preclinical studies and clinical trials, or the addition or termination of preclinical studies, clinical trials or funding support; |
| • |
the fluctuations in valuation of our derivative warrant liabilities; |
| • |
the timing of the release of results from any preclinical studies and clinical trials; |
| • |
an inability to complete product development in a timely manner that results in a failure or delay in receiving the required regulatory responses, approvals, or allowances to commercialize product candidates; |
| • |
the timing of regulatory responses, submissions, and approvals; |
| • |
the timing and willingness of any current or future collaborators to invest the resources necessary to commercialize our products; |
| • |
the outcome of any litigation; |
| • |
changes in foreign currency fluctuations; |
| • |
competition; |
| • |
the timing of achievement and the receipt of milestone payments from current or future third parties; |
| • |
failure to enter into new or the expiration or termination of current agreements with third parties; |
| • |
failure to introduce our products to the market in a manner that generates anticipated revenues; |
| • |
execution of any new collaboration, licensing or similar arrangement, and the timing of payments we may make or receive under such existing or future arrangements or the termination or modification of any such existing or future arrangements; |
| • |
additions and departures of key personnel; |
| • |
strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments, or changes in business strategy; |
| • |
if any of our product candidates receives regulatory, or fails to receive approval, market acceptance and demand for such product candidates; |
| • |
regulatory developments affecting our product candidates or those of our competitors; and |
| • |
changes in general market and economic conditions. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the market price of our common shares could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the market price of our common shares to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
There can be no assurance that an active market for our common shares will be sustained.
There can be no assurance that an active market for our common shares will be sustained. Holders of common shares may be unable to sell their investments on satisfactory terms. As a result of any risk factor discussed herein, the market price of our common shares at any given point in time may not accurately reflect our long-term value. Furthermore, responding to these risk factors could result in substantial costs and divert management’s attention and resources. Substantial and potentially permanent declines in the value of our common shares may adversely affect the liquidity of the market for our common shares.
Other factors unrelated to our performance that may have an effect on the price and liquidity of our common shares include positive or negative industry or competitor news; extent of analyst coverage; lessening in trading volume and general market interest in our common shares; the size of our public float; and any event resulting in a delisting of our common shares.
If we fail to meet applicable listing requirements, the NASDAQ Stock Market or the TSX Venture Exchange (“TSXV”) may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline.
Our common shares are currently listed on the NASDAQ Stock Market and the TSXV, but we cannot assure you that our securities will continue to be listed on the NASDAQ Stock Market and the TSXV in the future. In the past, we have received notices from the NASDAQ Stock Market that we have not been in compliance with its continued listing standards, and we have taken responsive actions and regained compliance.
If we fail to comply with listing standards and the NASDAQ Stock Market or TSXV delists our common shares, we and our shareholders could face significant material adverse consequences, including:
| • |
a limited availability of market quotations for our common shares; |
| • |
reduced liquidity for our common shares; |
| • |
a determination that our common shares are “penny stock”, which would require brokers trading in our common shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our common shares; |
| • |
a decreased ability for us to issue additional equity securities or obtain additional equity or debt financing in the future. |
We may pursue opportunities or transactions that adversely affect our business and financial condition.
Our management, in the ordinary course of our business, regularly explores potential strategic opportunities and transactions. These opportunities and transactions may include strategic joint venture relationships, significant debt or equity investments in us by third parties, the acquisition or disposition of material assets, the licensing, acquisition or disposition of material intellectual property, the development of new drug candidates, the sale of our common shares and other similar opportunities and transactions. The public announcement of any of these or similar strategic opportunities or transactions might have a significant effect on the price of our common shares. Our policy is to not publicly disclose the pursuit of a potential strategic opportunity or transaction unless we are required to do so by applicable law, including applicable securities laws relating to periodic disclosure obligations. There can be no assurance that investors who buy or sell common shares are doing so at a time when we are not pursuing a particular strategic opportunity or transaction that, when announced, would have a significant effect on the price of our common shares.
In addition, any such future corporate development may be accompanied by certain risks, including exposure to unknown liabilities of the strategic opportunities and transactions, higher than anticipated transaction costs and expenses, the difficulty and expense of integrating operations and personnel of any acquired companies, disruption of our ongoing business, diversion of management’s time and attention, and possible dilution to shareholders. We may not be able to successfully overcome these risks and other problems associated with any future acquisitions and this may adversely affect our business and financial condition.
We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies.
We are a “smaller reporting company” under the SEC’s disclosure rules, meaning that we have either:
| • |
a public float of less than $250 million; or |
| • |
annual revenues of less than $100 million during the most recently completed fiscal year; and |
| o |
no public float; or |
| o |
a public float of less than $700 million. |
As a smaller reporting company, we are permitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a smaller reporting company, the scaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies.
If investors consider our common shares less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common shares and our share price may be more volatile.
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We are a non-accelerated filer under the Exchange Act and we are not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our common shares less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and trading price for our common shares may be negatively affected.
We are a Québec incorporated company headquartered in Canada, and U.S. investors may be unable to enforce certain judgments against us.
We are a company existing under the Business Corporations Act (Québec). Some of our directors and officers are residents of Canada, and certain of our assets are located outside the United States. As a result, it may be difficult to effect service within the United States upon us or upon some of our directors and officers. Execution by U.S. courts of any judgment obtained against us or any of our directors or officers in U.S. courts may be limited to assets located in the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of U.S. courts predicated upon civil liability of us and our directors and executive officers under the U.S. federal securities laws. There may be doubt as to the enforceability in Canada against non-U.S. entities or their controlling persons, directors and officers who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities predicated solely upon U.S. federal or state securities laws.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
None.
| Exhibit No. |
Description |
|
| 101.INS |
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document |
|
| 101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
|
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
| 101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
| 104 |
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: November 10, 2021
| ACASTI PHARMA INC. |
|||
| By: |
/s/ Janelle D’Alvise |
||
| Name: Janelle D’Alvise |
|||
| Title: President and Chief Executive Officer and Director (Principal Executive Officer) |
|||
| By: |
/s/ Brian Ford |
||
| Name: Brian Ford |
|||
| Title: Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|||